Video-microscopy
and pictures of
Borrelia burgdorferi and other spirochete like structures links
collection
By
Marie Kroun, MD
Note:
If YOU have videos or pictures in the same category that you want to
show the
world, don’t hesitate to contact me at kroun (at) ulmar.dk
You might need to install certain
players / viewers for viewing certain types of files on your computer.
For
best print use papersize A4 and margins 10-15 mm!
Please also check out http://lymerick.net/MK-videomicroscopy.html
.. show more examples of MKs videomicroscopies for spirochetes, done
after 2006 without access to specific direct immune stain for
Bbsl, plus at lot more on the old research giving the rationale
for studying blood in the microscope.
Survival in Adverse Conditions (2001)
An
organized collection of photographs and quotations selected from
studies
finding evidence of alternate forms of various spirochetes, dating
from the
early 1900's through the present. Written permission to
publish
this
article in LymeRICK from the author, who wants to remain anonymous.
Alan MacDonald – USA
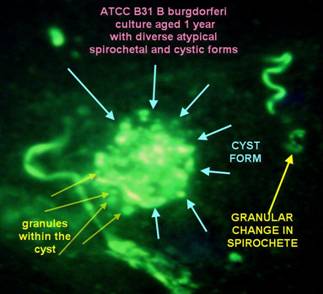
Culture of Borrelia burgdorferi (B31)
-
stained
with
fluorescin marked specific anti-Borrelia antibody;
done circa 1985
Visit
dr. MacDonalds
new website on Alzheimer
& Molecular biology / Borrelia
- read his answer to "why so many pictures?"
“Of Course you have my permission to include any of my images on your web site.
I
Do not
desire to copyright the images, because so many in the Lyme
Borreliosis community have viewed these images over the 20
years
since they
were created, that it constitutes a de facto acknowledgment
and I
know that
my photomicrographs exist to serve as tools for education, and not for
commercial purpose.
The image of the "cystic" form was obtained from very aged cultures
of B31 from the ATCC. After the motility of the borreliae ceased, the
"formes atypiqes" appear, and there forms are very diverse. Cystic
forms which contain granules inside of the cysts are just one of a
myriad
of
atypical forms that Bb may assume.”
[wrote
Alan MacDonald to Marie Kroun in personal e-mail about this picture on
20050830]
Matti Viljanen, MD, Professor – Finland

Tube phagocytosis of Borrelia burgdorferi
(fast
Internet
connection Real Player Movie 3 Mb.
Above
links to – and following is the text copied from –Viljanens own website:
”The video is a digitized version of the original video, from which the still images in figure 2 of the article "Tube Phagocytosis, a Novel Way for Neutrophils To Phagocytize Borrelia burgdorferi" by Juha Suhonen, Kaija T. Hartiala and Matti K. Viljanen (Infection and Immunity, July 1998, Vol. 66, No. 7) were captured.
The video is presented with the kind permission of American Society for Microbiology. The video presents a novel type of phagocytosis described in the article mentioned above. The original video was produced by observing and recording the interactions between human neutrophils and Borrelia burgdorferi, the Lyme disease spirochete, using dark-field microscopy with video technology. The digital version was made by Antero Lehtonen.”
Marie
Kroun,
MD – Denmark
 0018-GCS2.wmv
18 MB, 2
minutes video
first presented at the conference in York 2003 in this PowerPoint
lecture
0018-GCS2.wmv
18 MB, 2
minutes video
first presented at the conference in York 2003 in this PowerPoint
lecture
Microscopy of unfixed, unstained wet drop of peripheral blood from MKs pilot project patient #18
Case history was presented in MKs lecture in York, UK, 2004, presenting as leucocytoclastic vasculitis which has been found associated with Borrelia infection in a few cases!
This patient was first diagnosed with Borrelia burgdorferi plus tick borne co-infections with HME and Babesia like organisms back in December 2001!
This
video is from April 2003, i.e. about 16 months
after the
initial
diagnosis and despite intermittent antibiotic treatment with doxycyclin
for a
few months, and demonstrates a “pearls
on
a string”
spirochete like structure that can be seen
moving inside a
cellular
structure, possibly a white blood cell?
Despite bad
video quality, it should be
obvious
to everyone seeing this video, that it is NOT a NORMAL finding within a
white
blood cell to find a structure moving serpentine like - as illustrated
in this
video!

Since 2005 MKs videos has improved considerably, after she got a new handy video camera for USB – a Bresser PC-Microocular-II (cost only 99.95 Euro), which fits into the ocular of most microscopes.
A newer video of BUFFY-COAT fraction of blood of the same patient (#18) as above was taken 4 years after first diagnosis – 0018-20051213.wmv – shows persistence of similar blood changes compatible with persistent Borrelia infection, despite more oral courses of antibiotic treatment in between, leading to only temporary improvement in his clinical condition. Though obvious signs of vasculitis are rarely present nowadays – he still gets a few petecchiae now and then during his worst flares – he has many other fluctuating symptoms consistent with chronic Borreliosis, i.e. a chronic fatigue syndrome / ME-CFS like symptom presentation, like most other chronic Borreliosis project patients display!
A
RECENT VIDEO taken by MK with this camera was done on a fresh BUFFY-COAT wet blood drop (WMV,
50 Mb,
6 minutes): 0049-20060704.wmv
–
illustrates
spirochetes and granules.
Same pt. after 3 months treatment (WMV, 165 Mb incl. above movie): 0049-20061024.wmv
Samples
were taken the day after start of symptom flare on a male patient, who
was diagnosed
in Jan. 2006 with brain infarct and certain
neuro-Borreliosis,
i.e. by
being spinal antibody index positive for Borrelia burgdorferi and by
having
increased number of white blood cells in his spinal fluid,
and who
had improved
a lot, but only temporarily, on the usual two-week treatment
with
intravenous
ceftriaxon; he relapsed within a few months after stopping
antibiotic
treatment, was denied re-treatment because his neurologist thought he
had been
cured of Borreliosis because his Borrelia antibody response dropped
after
treatment! – after above confirmation of his relapse of active
Borreliosis, he
has started oral antibiotic re-treatment, has experienced a
Jarisch-Herxheimer
reaction within the first week of treatment and begin to feel
improvement
again, thus so far he follows the usual treatment pattern for chronic
Borreliosis.
So
far
(July 06) 48/48 patients (#42 was reserved, but has not been Bowen
tested yet)
with similar structures found in their blood by microscopy and
displaying
current symptoms compatible with having active Borreliosis, had
positive
outcome of a specific immune stain for Borrelia burgdorferi, done by
Bowen RTI,
see below!
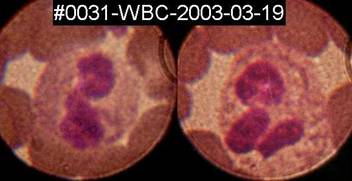 Spirochete
like structures can sometimes, but rarely, be seen inside white blood
cells,
here inside two granulocytes on a fixed, Diff-Quik stained blood-smear
from
patient#31.
Spirochete
like structures can sometimes, but rarely, be seen inside white blood
cells,
here inside two granulocytes on a fixed, Diff-Quik stained blood-smear
from
patient#31.
Thus
Marie
Kroun’s microscopy findings support the observation done by
others,
that Borrelia burgdorferi can
be located
inside WBC and possibly
also RBC, since not all the cellular structures with
moving
granules in
it contain a visible nucleus!
While
it
seems that any type of white blood cell – neutrophile, eosinophile (and
possibly also basophile?) granulocytes, as well as monocytes and
lymphocytes
judging from the appearance of the nucleus, can apparently harbour
moving
“granules” of spirochetes, it is also obvious to MK that it is not all
“granulated cellullar structures” that contain a nucleus!
Note that monocytes and lymphocytes, which have either a kidney shaped
or a
round nucleus without lobes, does not normally contain any granula in
their
cytoplasm, so when moving i.e. alive “granules” are found within the
“mononuclear” cell types, there is NO possible confusion about what is
normal /
abnormal “granules”, its clearly abnormal when found in
mononuclears!
More important, the spirochetal “granules” are usually larger and also
a bit
darker in appearance in phase contrast microscopy, than the normal
granula
found within the cytoplasm of the normal granulocytes, and last but not
least granula
within a normal granulocyte do NEVER move in a “pearls on a string” /
spirochete like fashion!
Thus
Borrelia – this master of disguise microbe – probably can use a wide variety
of host
cells as a
sheltered environment during its development, just like we
will
shelter a
newborn, weak baby in an incubator. This
is very far
from being a NEW OBSERVATION!
Already Balfour
in
Lancet 1911 described that granules
of fowl spirochetes
entered RED BLOOD CELLS
and Hindle
in 1912
drew spirochetes and granules inside nucleated cells found within the
Argas
tick!
Unstained wet-drop microscopy can be done on a fresh ear prick blood sample, on anti-coagulated full blood (EDTA, citrate) or maybe even best on sample from the Buffy-Coat fraction — see MKs how to make buffy-coat smear video – because as shown by MKs video samples from buffy-coat wet drop preparation, it appears to be ideal for finding (moving) “granulated cellular structures” and could be combined with use of a highly specific immune stain for any suspected pathogen, for more specific diagnostics, like done by Bowen RTI, however, quantification does not make any sense on buffy-coat fraction, of course!
Moreover,
the buffy-coat fraction is ideal material for finding many
other
blood
parasites, since both free microbes and immune cells, that have
phagocytised
microbes, fulfilling their normal task of trying to eliminate
foreign
intruders from the blood, will concentrate in the buffy-coat fraction,
see some references on
buffy-coat microscopy for parasites: QBC Malaria,
Paralens,
and Google
and
PubMed search.
While
wet
blood drop preparation is short-lived because the blood
cells lyses within
a
few days or hours, if contact with air is not avoided by
packing the edge of the cover glass for instance with Vaseline (avoid
contamination of the blood sample when applying), the buffy-coat blood smears can be stored
for several
years ahead
for later examination!
A German colleague and good friend, who is a tropical disease
specialist, gave
MK a 20-year old blood smear from a malaria patient; we stained it and
looked
in the microscope and really couldn’t tell that it wasn’t a recent
smear;
malaria parasites were easily visible in it, many more than is usually
the case
with the tick-borne cousin babesia!
Since
the cost of the glass slides are low, they don’t take up much space if
you
stack them in the same box you bought the slides in, and the smears can
later
be used for trying other stains for comparison, for using additional
(more
specific immune) stain or you might even scrape blood off the slide for
doing
PCR test in order to get a more specific diagnosis than just “ringforms
seen”
or “morulae-like inclusions seen”, or you might use it for education of
laboratory workers and colleague microscopists etc. –
THEREFORE ALWAYS
TAKE PLENTY
(buffy-coat) BLOOD SMEARS whenever you have the chance;
you
can really
never get too many good blood smears with parasites in it, and you may
have
only ONE chance per patient before treatment to catch some of these
parasites
of which there are usually few in relatively few of the chronically ill
patients with tick borne infections!
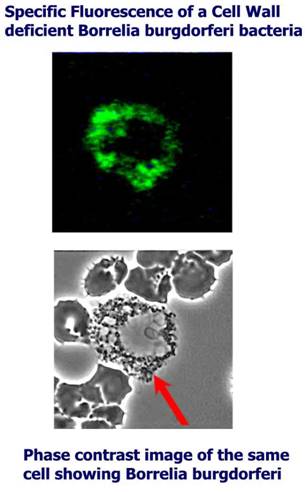
Dr.
Joanne Whitaker et al.
patented their procedure for detecting Borrelia burgdorferi ANTIGEN by
specific
fluorescent antibody stain in Jan. 2005, see
the US-patent (6,838,247) for all
details about their
Q-RIBb
method!
The
main advantages of Bowen RTIs Q-RIBb test are:
1. Documentation by picture(s) of any microscopic finding, so you can
compare
your own microscopy findings to that
2. Quantifying - by titration - the number of structures reacting with
added
specific antibody against Borrelia burgdorferi.
As
stated in the patent description Bowen
RTI use a
commercially available FITC-labelled anti-Borrelia burgdorferi antibody from Kirkegaard & Perry
Lab.:
Affinity purified polyclonal antibody to
Borrelia
burgdorferi made
in Goat and labeled with fluorescein isothiocyanate (FITC). Isolated
from a
serum pool of goats immunized with heat killed whole cells of Borrelia
burgdorferi. The
antibody is highly specific for Borrelia burgdorferi. Cross
reactivity to Borrelia hermsii, Borrelia coriaceae, and Borrelia
anserine has
been minimized through extensive affinity adsorption. Product
is in
lyophilized form. Each lot is tested to assure specificity and
lot-to-lot
consistency using KPL's in-house ELISA assay.
Andy Wright, MD – UK
 AndyWright2004-640.wmv (52 Mb, 4
minutes) video of spirochetes in more
lenghts, granules,
a moving granulated cellular structure,
thus illustrating all the phases complex spirochetal lifecycle drawn on
page 475 in this article by Hindle 1912 (PDF)
printed in Parasitology (1912), iv, pp 463-477.
AndyWright2004-640.wmv (52 Mb, 4
minutes) video of spirochetes in more
lenghts, granules,
a moving granulated cellular structure,
thus illustrating all the phases complex spirochetal lifecycle drawn on
page 475 in this article by Hindle 1912 (PDF)
printed in Parasitology (1912), iv, pp 463-477.
So far (June 2005) 98/98 of Andy’s ME/CFS patients
with such structures in their blood tested positive on direct
fluorescent
antibody test for Borrelia burgdorferi ANTIGEN!
Bela Bozsik, MD – Hungary
 Dualdur®
movie
Dualdur®
movie
70
Mb, 11
minutes dark-field microscopy video of Borrelia
burgdorferi
spirochetes
in blood, showing the shedding of ‘granules’, budding of the spirochete
at the
end and centrally, the formation of blebs and transversal division of
the
spirochete. Dr. Bozsik adds a reagent to the blood named Dualdur, on
which he
holds the patent, which immobilizes blood cells, but do not disturb the
spirochetal movements.
More about Bela Bozsik: http://lymerick.net/BozsikBP.html ... Dual-Dur patent: 6689577 or Wikipatents, USPTO
Dr. Bozsik kindly gave me his permission to present both his lectures - DIAGNOSIS and THERAPY – held at the Sheffield 2005, UK LDA conference on tick-borne infections
Both
presentation are made in PowerPoint 2003; some embedded videos from
“The
Passion of Christ” and “biting tick” shown at the conference, does not
seem to
work properly from within in this PowerPoint presentation
when online?
The
latter - tick
bite video -
show us how an Ixodes
tick enters
its hypostoma (blood suction organ) directly into a small skin capillary.
The
implication is that when the tick has Borrelia burgdorferi in its
salivary
glands – some 5% to as many as 35% of
examined
ticks have been reported to be
“systemically infected” with Borrelia and these
ticks
already harbour spirochetes in their
salivary glands and thus do NOT need about 24 hours for becoming
infective
– they
might transmit
Borrelia infection immediately when they bite, as the spirochetes will
be
injected directly into the blood-stream with the ticks saliva!
Thus we must all realize by simple logic that Borreliosis
must always be a blood infection right from start,
explaining
how
spirochetes
can spread
very early into all tissues and compartments, like
the central nervous system within only two weeks of the tick bite
– weeks
before or
without any development of concurrent ERYTHEMA
MIGRANS which is NOT, as it is usually described, a
PRE-NEUROBORRELIOSIS manifestation,
but is to be considered a possible SIDE MANIFESTATION of Borreliosis,
possibly
already having spread hematogenously, which is fully consistent with
the fact
that a previous or simultaneous Erythema migrans is recorded in just
about 50%
of cases that present with neuro-Borreliosis!
The implication of recognizing the possibility of EARLY SPREAD is that any antibiotic treatment for Borreliosis should always aim to reach a sufficient concentration, i.e. over the minimal inhibitory / bactericidal (MIC/MBC) concentration, within the central nervous system (CNS) and other deep tissues, also for early Borrelia infection, i.e. also when “just” the telltale sign of Borreliosis: Erythema migrans is present!
– otherwise there is a potential risk that some slightly more antibiotic resistant Borrelia may have spread to and can survive in relative undertreatment within the CNS or in other deep tissue compartments, from which a later bout of active Borreliosis could come, that can be much harder to treat effectively, due to selection of more antibiotic resistant variants because they survived at suboptimal antibiotic concentration may need higher dosis next time in order to be effective!
Many
published
reports on cases of
progression
of symptoms / relapses of Borreliosis after early, but low dose and
short time
antibiotic treatment for erythema migrans (usually with tablet
penicillin or
low dose doxycyclin given for only 10-14 days), show us that this risk
is not
just a hypothetical concern!
Dr.
Bozsik found –
data
extracted from his Diagnosis lecture–
that:
- Spirochetes could be demonstrated in BLOOD by dark-field microscopy during all ACTIVE stages of pathogenesis!
- 107
of 143 (75%) of the
cases with live
(moving) spirochetes found in their blood by dark-field microscopy,
were confirmed by real-time PCR to belong to Borrelia burgdorferi sensu lato:
66 (61.7%) B. burgdorferi sensu stricto
20 (18.7%) B. garinii
6 ( 5.6%) B. afzelii and
15 (14.0%) other Borreliae strains than the usual EUROPEAN strains
Many of these cases were also confirmed by monoclonal antibody stain for Borrelia burgdorferi with anti-ospA and anti-flagellin kindly donated by prof. Barbour USA. - One third (1/3) of these patients with proven LATE Lyme borreliosis were SERONEGATIVE!
MORE ABOUT SERONEGATIVE BORRELIOSIS:
A
considerably high number of patients with proven late Borreliosis, i.e.
where
persistent Borrelia infection was proven by ANTIGEN DETECTION - either by CULTURE,
PCR and/or MICROSCOPY (sometimes aided by specific immune stain) for
Borrelia
burgdorferi
- has unfortunately been shown to test SERONEGATIVE on a wide
variety of
BORRELIA SEROLOGY tests – as demonstrated for instance in the reports
listed in
the following section.
Oksi et al. J Clin Microbiology 1995 Sep; 33(9): 2260-4 (PDF):
·
All
41
patients had symptoms of Borreliosis for at least 3
months
i.e. were
LATE Borreliosis cases.
78.0% were sick for >= 6 months, 53.7% were sick for over 1 year!
"…. multiple organs were frequently involved. Recurrent
fever
episodes were seen in nearly half of the patients, neurological
symptoms were
seen in more than half of the patients, and musculoskeletal
manifestations were
seen in three-fourths of the patients. Moreover, most of these
manifestations
were long-lived. In spite of this, several patients were seronegative
and most
seropositive patients had only weakly positive antibody levels.”
·
All
patients were positive for Borrelia burgdorferi ANTIGEN:
12 were positive on CULTURE, 39
were positive on
PCR
– 10 were positive on both CULTURE & PCR!
·
Performance
of 3 different SEROLOGY tests all used on all 41 patients:
…
data from
table 2, page 2261;
however, MK added % calculation and text enhancements to the table:
Sensitivity (~ true positives in patients) and specificity
(~ true
negatives in controls) are marked with bold and black.
False positive
(in
controls) and false
negative
(in patients) are marked with bold
and red.
|
|
SA-ELISA
IgM, IgG, or both |
FL-ELISA
IgM, IgG, or both |
P39-ELISA |
|||
|
|
Positive* |
Negative |
Positive* |
Negative |
Positive* |
Negative |
|
Culture positive (n=12) |
11 26.8% |
1 2.4% |
6 14.6% |
6 14.6% |
5 12.2% |
7 17.1% |
|
Only PCR positive (n=29) |
21 51.2% |
8 19.5% |
11 26.8% |
18 43.9% |
1 2.4% |
28 68.3% |
|
Total patients (n=41) |
32 78.0% |
9 22.0% |
17 41.5% |
24 58.5% |
6 14.6% |
35 85.4% |
|
Controls (n=37) |
4 10.8% |
33 89.2% |
5 13.5% |
32 86.5% |
2 5.4% |
35 94.6% |
*
Positive
results include weakly
positive, positive as well as strong positive results!
Note
that %
of patients who had true
positive serology with P39-ELISA was less than the chance of hitting 6
when
throwing a dice (1/6 ~ 16.7%)!
·
Overall
results of ALL 3 SEROLOGY tests on ALL 41 patients:
Sensitivites (true positives) for combined tests:
SA-ELISA + FL-ELISA: 80.5%; SA-ILISA + P39-ELISA: 80.5%;
FL-ELISA +
p39-ELISA: 51.2%
SA-ELISA + FL-ELISA + p39-ELISA: 82.9% - thus for only 34 of 41
patients, the
diagnosis could be confirmed by positive serology, thus
7 of 41 (17%)
patients were
SERONEGATIVE on ALL THREE serology test, despite having proven LATE
Borrelia
infection!
·
Although
ALL patients suffered from symptomatic
LATE LYME BORRELIOSIS, 18 (43.9%) had ONLY
positive
Borrelia-IgM,
i.e. were Borrelia-IgG negative, respectively 10 (24.4%) on
SA-ELISA and 8
(19.5%) on FL-ELISA.
Both FL-ELISA and P39-ELISA detected only one positive specimen which
had gone
undetected by the other two tests.
Eldøen et al. Tidsskr Nor Lægeforen 2001; 121: 2008-11 (PDF in Norwegian):
·
14 of 25
(56 %)
patients with spinal index positive (~certain) NEUROBORRELIOSIS had
specific
borrelia-IgM and IgG-antibodies ONLY in their spinal fluid, while their
- at
same time as spinal fluid sampled - SERUM samples were negative for
Borrelia-antibodies.
The Norwegian laboratory used two different serology tests, one of
which is the
above mentioned FL-ELISA (DAKO)!
The
14
FALSE SERONEGATIVE patients would probably have gone undiagnosed and
untreated
for their certain neuro-Borreliosis, if their Norwegian doctors had
relied ONLY
on their false negative SERUM Borrelia antibody result as been a 100%
reliable
diagnostic measure to out-rule the possibility of neuro-borreliosis, as
many
doctors are unfortunately still in the habit of doing!
… HMMM when serology test overlooked over half of the truly very sick
patients
in their SERUM samples, how many patients could have been missed on
false
negative spinal fluid ELISA’s, perhaps another 50%? ..
Lomholt H et al. Acta Derm Venereol 2000 Sep-Oct;80(5):362-6 (PMID: 11200835):
·
From
PubMed
abstract: “The
kinetics of antibodies to Borrelia burgdorferi following successful
treatment
of early and late cutaneous borreliosis were analysed in consecutive
serum
samples by an enzyme-linked immunosorbent assay (ELISA) technique.”
[Danish
study, so it was probably FL-ELISA from DAKO, Glostrup!?]
“Twenty-three patients with culture positive erythema migrans
were
followed for 23+/-14 months: 41% stayed
seronegative,
35% showed an
isolated
immunoglobulin M
(IgM) response,
8% an
isolated IgG response and 16% a combined IgM and IgG
responses. …. In general, antibody levels peaked within the first 3
months of
symptom onset, whereafter a gradual decline was observed within 1 year.
Treatment success may in part be monitored serologically for both
seropositive
erythema migrans and chronic cutaneous borreliosis as most patients
show
declining titres after successful treatment. However, continuously high
titres
do not necessarily indicate treatment failure.”
Oksi et al. Ann Med 1999 Jun;31(3):225-32. (PMID: 10442678)
· 32/165 = 19,4% had clinical relapse after more than 3 months antibiotic treatment for borreliosis.
· In 13/32 (40,6%) could the relapse be verified by either positive PCR (12) and/or positive culture (3) for B. burgdorferi
· At time of proven relapse 6/13 (46%) were seronegative!
· 12/13 were seropositive at initial diagnosis, i.e. 5 pts. developed seronegativity despite proven persistent borreliosis!
· 5/13 (38%) had circulating immunecomplexes, of these 3 were seronegative.
·
1
patient (10) was seronegative throughout the whole course of illness
despite
both positive culture and PCR in CSF primarily, and positive biopsy and
plasma
PCR for Borrelia burgdorferi at time of relapse!
This patient had been treated with ceftriaxone IV 2g for 3 weeks,
followed by
24 weeks of doxycycline 100 g bid and amoxicillin 1 week - a total of
28 weeks
(6-7 months).
· 1 patient (8) had been treated for as long as 47 weeks (11 months) including 7 weeks of intravenous ceftriaxone - primary diagnosis was confirmed by positive biopsy and the relapse 44 weeks after treatment confirmed by a positive plasma PCR for Bb.
· 1 patient (2) had relapse 130 weeks after 1. treatment, that had lasted 16 weeks. Pt. was seropositive initially (both IgM and IgG), but seronegative at time of relapse; the relapse was confirmed by positive PCR for Bb, no history of re-infection in the meantime.
Marie Kroun’s pilot-project on patients all highly suspect for chronic Borreliosis, presented at medical conferences in UK and PowerPoint presentations from 2003 and 2004 presented in LymeRICK website:
·
36%
of 33
patients
tested
SERONEGATIVE on the danish serology test for
Borreliosis (FL-ELISA, DAKO) despite all presented with abnormal
structures in
their blood, as shown in above videos, and similar structures were
confirmed to
be Borrelia burgdorferi related, by positive stain in Bowen RTIs (Q-)
RIBb test
for Borrelia burgdorferi!
… more follows from the
long term
follow-up project in due time
· It was a similar case as in the Norwegian study that started MKs special interest for Borreliosis back in 1994; a 9-year old boy that was highly suspect of Borreliosis due to severe burning pains in both his legs, but who tested SERUM negative for Borrelia antibodies more times, a factor leading to delayed spinal puncture, which, when finally done, showed SPINAL FLUID was HIGHLY Borrelia ANTIBODY positive by FL-ELISA, while at the same time sampled BLOOD SERUM was SERONEGATIVE and the boy was still SERUM ANTIBODY NEGATIVE for Borrelia 4 months later!
THESE AND MANY OTHER PUBLISHED WORKS ALL EMPHASISE THAT ANY NEGATIVE
BORRELIA SEROLOGY TEST CAN NOT BE USED TO
EXCLUDE PERSISTENT
ACTIVE
BORRELIOSIS in the SYMPTOMATIC PATIENT – not
even when more
SEROLOGY
tests are being used in combination!
Thus a
declne in antibody result after antibiotic treatment,
SHOULD NOT be taken as a positive sign of the Borrelia infection was
cured
forever, especially not when the patients symptoms recur shortly!
– when symptoms reappear within
less than 1-3 YEARS after stopping antibiotic treatment for proven
Borrelia
infection, then suspect a relapse of ACTIVE Borreliosis and seek
proof of it
by all possible means!
When
suspecting RELAPSING or CHRONIC PERSISTENT ACTIVE
BORRELIA INFECTION – forget using ANTIBODY tests as the first
diagnostic
measure!
…
the only proper use for Borrelia serology is that it can sometimes help
prove
EARLY Borrelia infection, since a negative serology test sometimes but
unfortunately not always turns positive a while into the Borrelia
infection.
Serology is of NO PRACTICAL USE in LATE Borrelia infection, because
1. multiple serology test were FALSE negative in several culture, PCR and/or microscopy proven cases of highly symptomatic Borreliosis patients, that usually showed benefit from antibiotic treatment at least as long as it was being given! – and because
2. serology can persist positive for years in asymptomatic individuals with latent Borrelia infection, who won’t benefit from antibiotic treatment, unless their latent infection begin to show signs of renewed ACTIVITY, i.e. the patient becomes symptomatic again! … and because
3. serology results can sometimes be “false positive”, due to cross reaction with other bacteria with similar antigens
Hence serology bear both a very high risk of undertreatment and of overtreatment ..
PLEASE
NOTE THAT UNDERTREATMENT
of Borreliosis is MUCH WORSE for the patient
than "OVERTREATMENT" of clinically suspected persistent Borreliosis,
because the side effects of antibiotic treatment are usually
neither severe nor longer lasting - while letting Borreliosis go on
untreated can be debilitating and even deathly for the patient in
question!
INSTEAD
OF
SEROLOGY DO FULL BLOOD MICROSCOPY (preferably of
buffy-coat fraction), which any doctor with a microscope could quickly
learn to
do by looking at the video's on this website!
- and if found changes like shown in the video’s and pictures above,
TRY TO
CONFIRM BY SPECIFIC ANTIGEN TESTS, whenever possible!
It is important to try to confirm the clinical diagnosis with more
ANTIGEN
tests, because finding any positive ANTIGEN test (in opposition to a
positive
ANTIBODY test) helps justify trial treatment with antibiotics
and also justify
the need for social economic support, when the patient suffers from so
many
invalidating symptoms, documented in the patients symptom
diary, that
can prevent her/him from working for a long (sometimes life-long)
period of
time due to persistent ACTIVE Borreliosis!
CLOSING REMARKS:
Because
all spirochetes seem to go through all the same changes during
their developmental cycle, as demonstrated in Survival
in Adverse Conditions (2001) –
any spirochete would possibly give about the
same
microscopic
picture … thus
it is important to understand
that wet
blood drop microscopy can NOT ALONE be used to diagnose Borrelia
burgdorferi
infection specifically; diagnosis made by wet blood drop microscopy as
shown by
MK can ONLY be “suggestive of spirochetosis”!
So should you ever happen to see similar
(moving)
“granulated cellular structures” as shown in the videos on this
website, in an
unstained wet blood drop microscopy or you see spirochete like
structures
situated within blood cells in a stained blood smear or see spirochete
like
structures moving in plasma (outside cells), then you
should always do supplementary SPECIFIC ANTIGEN DIAGNOSTICS, like doing microscopy with specific immune
stain for B.
burgdorferi, PCR and/or try to culture the microbes for confirmation /
typing
of the spirochete!
Any other possible cause for any of the patient symptoms / clinical signs – like myxoedema (hypothyroid and other hormone disturbances and autoimmune phenomena are often found to accompany (is it caused by?) LATE Borreliosis) causing both chronic fatigue, mental depression etc. – should of course be found and addressed properly too, if found in the patient!
It is important to understand that Borrelia carriers without recurrent relapses in their history probably won’t benefit much clinically from antibiotic treatment, as long as their microbes still reside dormant, inactive within their deep tissues, i.e. as long they are being asymptomatic!
Dr. Bozsik estimates that 10% of all people in Europe might carry Borrelia burgdorferi, see his comment in The Lancet 2004; 363:901 (full text after free registration and login); most of these carriers are probably feeling healthy most of their life, i.e. are not feeling chronically ill from their persistent, but luckily for them INACTIVE, latent persistent Borrelia infection!
It is important to understand that Borrelia burgdorferi can only be hit by antibiotics, when they are active and proliferating! – i.e. during the patients SYMPTOMATIC PERIODS! – which can be judged ONLY by patients detailed symptom log, especially if it shows the for Borrelia typical cyclical pattern repeatedly! – combined with multiple tests able to diagnose ACTIVE Borrelia infection specifically, and other test done to out rule other possible causes of the patients symptoms!
Compare the Borrelia carrier situation to the well known fact that any person who in childhood once had a primary herpes simplex (cold sore) or varicella infection (chicken pox) infection – and this applies to about 80% of all adults being seropositive for herpes simplex and about 90% being seropositive for herpes varicella-zoster! – will for the rest of their life carry the viruses within them and thus will carry the potential for later developing a usually localized eruption - AKA cold sore or herpes zoster / shingles respectively – when/if their immune system weakens and is no longer able to suppress the growth of the virus. Most herpes infected individuals never get a clinical relapse, i.e. cold sore or shingles respectively! – but we could probably be lucky to sometimes detect herpes virus DNA within their tissue, if sampling for it was done in the right place at the right time! Luckily herpes virus growth can nowadays be effectively hindered by treatment with acyclovir, while acyclovir has no positive effect whatsoever on dormant inactive virus and can thus not prevent future relapses! It is well known that herpes simplex usually flare up in hosts otherwise asymptomatic whenever the carrier gets a common cold or another infection, or after a period of extreme stress (both physical and psychological), and when if the host lack nutrients, sleep i.e. lives an un-healthy life style. Just like herpes virus – all spirochetes may have long periods in which the microbes are dormant AKA hibernating, latent, asymptomatic infection, during which the metabolic processes that antibiotics can interfere with are all idle, thus antibiotic treatment given during inactive periods will probably be wasted like acyclovir for herpes! – but can be useful when latency is followed by periods of flare up!
Borrelia can – like herpes virus – be kept at bay for long periods by a well functioning host immune system probably for decennials or even for the rest of that individual’s life, without causing any symptoms at all?! – but may flare up anytime whenever host conditions i.e. lower immune defence allows it, once Borrelia has been introduced into the host!
We could therefore possibly also possibly sometimes detect “a few” Borrelia ANTIGEN during “small flares” on samples from the Borrelia carrying host, that is asymptomatic most of the time, i.e. who is not chronically ill, who probably won’t benefit from antibiotic treatment. Hence testing asymptomatic persons is NOT relevant; test ONLY SYMPTOMATIC PATIENTS!
While absence of or disappearance of a previously present specific immune reaction in the patient with known (proven) Borrelia infection, seems to be a rather bad prognostic sign judged from many published and unpublished case reports! – it could probably be taken as a good prognostic sign, when a well functioning immune system recognize and keep up producing antibodies towards Borrelia long term after the acute stage, because the antibodies against the motility organs – FLAGELLA – probably hinders Borrelia mobility, hence hinders further spreading?!
It should therefore not surprise anybody, really, that the Borrelia patients so far studied which have focused on the SEROPOSITIVE INDIVIDUALS, show a relatively good prognosis, while those individuals with signs of (Borrelia specific) immune depression, such as total absence of, or only an immature response i.e. IgM only, i.e. no memory cells knowing how to produce IgG during later relapse .. and who often shows declining specific antibody-response after treatment despite ongoing Borrelia activity measured by both RECURRING SYMPTOMS and ANTIGEN TESTS, as illustrated in a very well examined case by Haupl et al.
Patients who turn out to have a very HIGH NUMBER of Borrelia burgdorferi related structures in their blood (high titer on Q-RIBb) taken when they are highly symptomatic WITHOUT SHOWING FLAGELLA ANTIBODIES against Borrelia, should LOGICALLY turn out to have a worse long term outcome than the first mentioned SEROPOSITIVE patient group!
Two very different patient groups with differing need for doctors assistance and follow up!
So relapsing patients – especially those who show absence of or declining Borrelia antibody level, despite being persistently symptomatic or who relapse shortly after stopping antibiotic treatment and who has shown (moving) “granulated cellular structures” in their blood during symptomatic period – should be followed long term (at least 5 years!) and should have very liberal access to re-testing for Borrelia ANTIGEN and antibiotic treatment, whenever they feel the need, i.e. when/if their symptoms relapse / develop further! Since antibiotic treatments generally has a low frequency of serious unwanted side effects, and because the disease caused by ACTIVE Borrelia burgdorferi and not the least the toxin(s) that Borrelia can produce when they are ACTIVE (causing worst symptoms when they are MANY bacteria present in fierce competition for low supply of nutrients and space) can be quite severe, life-long, debilitating and even lead to premature death in some cases! – trial antibiotic treatment should not be withheld from any symptomatic patient suspect of Borreliosis, even though certain Borrelia infection has not been proven by ANTIGEN tests yet!
A positive response to trial antibiotic treatment should always be taken as a sign that the patient has an ongoing infection that is treatable by antibiotics, whether is has been proven to be due to ACTIVE Borreliosis or not! The SYMPTOMATIC patient should NOT be denied further antibiotic treatment, because symptoms can be abrogated, just like when treating herpes with acyclovir, and because perhaps long lasting debilitating symptoms can possibly be prevented by prompt treatment every time the patients Borreliosis flares up?!
If a patient improves (again and again) on given antibiotic treatment, despite the doctor can perhaps not put a name to the infection yet (because the right test weren’t done at the right time)! – the patients physician should see the antibiotic-responsiveness as sign that this patient needs further testing AND offer treatment until the patient is no longer symptomatic!
IF the treating physicians would just CARE to follow and test these patients thoroughly for Borreliosis and co-infections with ANTIGEN tests, which is NOT DIFFICULT, as illustrated in this article! – and treat them for ACTIVE INFECTIONs, instead of mocking these patients by stating FALSELY that “all Borrelia was killed after 10-14 days”, and calling them “hypochondriacs”, I’m quite sure many cases of chronic Borreliosis, long term suffering and disability could be prevented!
Despite improved diagnostic methods, like by doing live video-microscopy / plus specific immune stain for Borrelia, in order to document the presence of spirochete(s) and/or their alternative forms, during all active stages of Borreliosis, the diagnosis of Late (chronic) Borreliosis remains – probably forever – a CLINICAL DIAGNOSIS, that can sometimes, but not always be SUPPORTED by any POSITIVE TEST RESULT, but can NOT BE OUT RULED by any NEGATIVE TEST RESULT! – so LISTEN to the patients, let them write SYMPTOM DIARY!