Buffy coat / Microhematocrit technique
- why use upconcentration by centrifugaton, followed by
light and fluorescent microscope
examination, using more staining techniques for
visualizing microbes?
By
Marie Kroun, MD (MK), 2012
With
use of the microhematocrit or buffy-coat technique,
i.e. by
centrifugation of the anticoagulated full blood sample, it is wellknown
from articles dating back to the 1970ies (see references below), that
free microbes in the plasma, like borrelia, trypanosoma, microfilaria,
other? .. will
accumulate in the platelet rich plasma fraction, just above
the
white buffy-coat layer (layer of white blood cells, i.e. immune cells),
while the red blood
cells
with intracellular organisms inside, like malaria or babesia,
will
accumulate in the layer of red blood cells just
below the
buffy-coat layer:
After
centrifugation of an anticoagulated (EDTA or citrate) whole blood
sample, wet drops (1 blood drop ~ 50 microliter; calculated from 20 drops per ml)
can be examined directly, unstained or stained with immunofluorescent
stain, under a cover glass, or can be smeared onto an object
glass, dried and stained as described below.
From the around 0,5 ml of buffy-coat fraction
gained per 5 ml blood sample, can be made 10-20 bloodsmears, and any residual material is frozen in the syringe, could perhaps be used for confirming PCR
test, if sign of microbes are found by the microscopy? - see video on
how
buffy-coat smear preparation is made. Dried blood smears has a very
long durability (20+ years) when stacked closely together
well protected. I cover the upper smear in a stack with a
clean object glass with ID date on a label, and fix the stack closely
together with tape. If not stored properly the dried blood may be accessible to insects i.e. "silverfish" (Lepisma saccharina) that like to eat it. After drying, the blood smears can be fixed and stained by any
conventional hematology blood stain, or a
Romanovsky stain type, like Diff-Quik: http://en.wikipedia.org/wiki/Diff-Quick
(which is easy and fast) or preferably by a microbe specific immunestain
method, if one has access
and money to buy relevant target specific antibodies, or
the detection of DNA/RNA can be enhanced by staining with acridine
orange and by using a fluorescence microscope...
Acridine orange:
http://en.wikipedia.org/wiki/Acridine_orange
QUOTE: "Acridine
orange binds to nucleic acids of cells and bacteria [DNA, RNA]. When
viewed under UV light, single-stranded
DNA and RNA fluoresce orange, whereas double-stranded
DNA appears green. At low pH (3.5 – 4.0), bacteria
and fungi stain bright orange.
Cellular
material stains pale green to yellow.2 Nuclei of activated
leukocytes may stain orange, yellow or red, depending upon the degree
of increased RNA production.
Erythrocytes either have no color or appear pale green."
Acridine Orange for
malaria diagnosis:
its diagnostic performance, its promotion and implementation in
Tanzania, and the implications for malaria control (2002)
http://www.cytoscience.com/acridine%20orange%20test%20keiser%20et%20al.pdf
"The
literature on the discovery, development and validation of the AO
[acridine orange] method for malaria diagnosis is reviewed here."
"The
basic feature of fluorescent staining is that, in contrast to the
mature erythrocytes that do not contain DNA or RNA, the nucleic acids
of the parasite fluoresce strongly."
"After testing 30 different
fluorescent dyes, Fuhrmann (1962) found an AO solution with a pH
between 6 and 7 and a concentration of 1: 10,000 to be the most
promising dye for the staining of thin bloodsmears containing Babesia
canis, P. berghei or P. cathemerium. ... shortly thereafter,
excellent
results were reported for the AO staining not only of thin smears
containing P. berghei or P. vivax (Ambroise-Thomas et al., 1965) but
also of thick smears containing various Plasmodium species (Sodeman,
1970). In the latter study, the thick smear was made slightly thinner
than was then customary and dehaemoglobinized during staining with a
0.01% AO solution of pH 5.4 (Sodeman, 1970). Commonly, AO
stain is applied to a thin smear only after the smear has been dried
and then fixed in methanol. However, an immediate method, in which AO
solution is applied directly to an unfixed and undried blood film, has
also been described (Kawamoto, 1991 a; Metzger and Nkeyi 1995)".
Fluorecens microscopy was for a long time too expensive
but ... "In 1991, however, the
development of a relatively cheap, interference-filter system (a
multi-layered excitation filter combined with a barrier filter)
designed for use with AO in a standard light microscope provided a
low-cost tool for malaria diagnosis employing AO
(Kawamoto, 1991 b).
A
combination of centrifugation, AO staining and fluorescence microscopy
was developed into the quantitative buffy coat (QBC) method. Although
this technique appears to be easy to handle, sensitive and rapid, it
requires costly pre-prepared tubes, a centrifuge and an ultra-violet
microscope (Lema et al., 1999). It is not being discussed here in more
detail."
Tick-Borne Relapsing Fever Imported from West Africa: Diagnosis by
Quantitative Buffy Coat Analysis and In Vitro Culture of Borrelia
crocidurae. (1999)
Full text:
http://jcm.asm.org/content/37/6/2027.full
PDF
"TBRF was
rapidly
diagnosed for two patients returning from western
Africa with fever of unknown origin by quantitative buffy coat (QBC)
analysis. ... By QBC
analysis of blood from both patients, brightly
luminescent spirochetes were observed (Fig.1),
concentrating at
the plasma-leukocyte interface. Thick blood
smears
were stained with Giemsa’s stain, and 200 oil immersion fields (×1,000)
were systematically examined. In thick smears from the second patient,
spirochetes were visible, whereas thick smears from the first patient
were negative, even after extensive reexamination."
In the QBC
method (see drawing above), by using a capillary tube which comes
pre-coated inside with acridine orange and anticoagulant,
and where a plastic float forces all the
surrounding cells
into the 40 micron space between its outside circumference and the
inside of the tube, any orange fluorescence in the top of the
erythrocyte layer under the buffy-coat layer, makes it faster and
easier to detect, if there are any parasitized RBCs, like malaria or
piroplasma (babesia) in the sample ...
QUOTE:
"Studies have shown that the QBC Malaria Test is 5.5 to 7 times more
sensitive than Giemsa thick films. The QBC Test also offers equivalent
specificity with a negative predictive value of greater than 98%. The sensitivity of the QBC
Malaria Test is particularly notable in cases of low parasitemia, as
the test allows for the detection of as little as 1 parasite per µL of
blood. In one study of 49 patients with low
parasitemia (defined as <10 parasites per µL of blood), the QBC
Test established diagnosis earlier than thick film in 47% of cases."
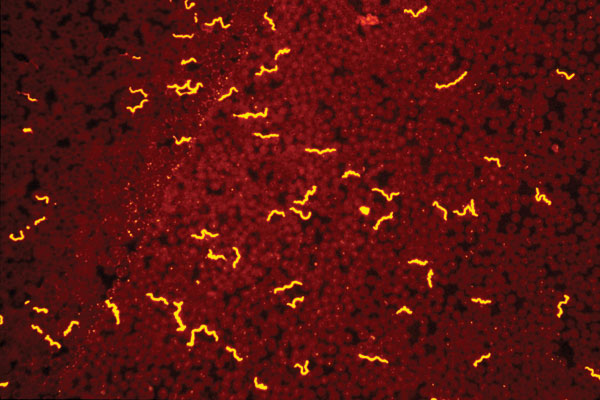
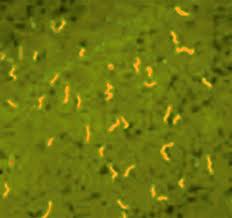
How
does Acridine Orange stain enhance detection of spirochetes
and parasites? - some illustrative pictures show it:
Leucocyte total and differential
count should be done on an unconcentrated sample i.e. thin blood
smear from capillary finger or ear prick or anticoagulated venous blood, since that gives additional information of any ongoing blood
disease. The RBC overview picture may show many cells of abnormal shape
(like acanthocytosis, ecchinocytosis), polychromasia (immature
RBC which has
lost
nucleus stains darker bluish, due to content of RNA, compared
to mature RBC), hypochromia (less red stain, sign of anemia), increased number of red cells with Howell Jolly bodies
inside etc. ... you need to know what all this looks like, so you better have a hematology picture atlas ready at hand to
compare what you see with, when beginning to do blood microscopy!
When looking at peripheral blood cells - http://lymerick.net/Blood-Cells.pdf
- the microscopist must be well aware of all the different
blood
cell types and of the dynamics of the blood cell formation in the bone
marrow, i.e. that in response to any increased use, i.e.
loss of
blood cells, the bone marrow will normally respond
by producing many more new blood cells and will send more new
immature cells (blasts .. myelocytes .. immature WBC),
normoblasts (immature RBC, still containing a nucleus) into the
peripheral
circulation, compared to what is seen under normal i.e.
non-disease,
condition
- hence the presence in
peripheral blood of increased number
of immunature blood cells, indicate increased production as sign of
increased use / loss of blood cells .. the cause of
this must
be
searched thoroughly for - of course!
Normoblasts
is a very rare finding in normal adult blood, so if one spot just one of
these,
think blood loss and lookout for possible cause: bleeding,
hemolysis, parasites?
In grave infection the WBCs will usually show signs of activation, enzyme production i phagocytes result in vacuolization and in toxic granulation of the granulocytes etc. In very severe infection the number of immature white blood in
peripheral blood may be so
high that it may nearly mimic a leukaemia blast crisis, it is called
leukemoid reaction. When you look at blood smears you might occasionally encounter a leukaemia
patient and should be able to differentiate!
The level of parasitaemia (% is the number of infected blood cells out
of 100 blood cells counted in a peripheral thin blood smear) can be so low, that the patient does not develop anaemia,
nor are there any overt sign of hemolysis in the urine when the haptoglobin
binding capacity for hemoglobin is not yet reached, luckily no
risk of kidney
damage at so low microbial level. It
is the immunreaction raised against the infection that cause disease
symptoms, more than the parasites themselves, and since the first line
of defence is complement activation and cytokine storm, that are
cascade reactions, just a few bugs can sometimes elicit a huge innate
immune response. Compare to an allergic reaction, where minute
amount of an allergen in the sensible person, can elicit a
deathly anaphylactic shock reaction, or in toxic shock syndrome,
where toxins are formed by bacteria growing on the mucous membranes,
can be absorbed through the membranes and elicit a deathly
cytokine response, despite there is not even an invasive blood
infection.
Hemoglobinuria detectable on urine stix may first appear at about 1%
parasitaemia
and visible hematuria - blood pis - may occur at
about 4+%
parasitzed cells (is sign of glomerular damage?) in human Babesia
infection, judged from reading pulished literature. Many
low-level Babesia carrier patients have less much than 1% of their red
blood
cells
infected (more like only 1 in a 1000 cells infected), i.e. the
parasites can be quite difficult to detect by
conventional thin and thick blood smear technique and even PCR; as
illustrated herein a higher sensitivity and less time to
first detection is achieved by upconcentration the blood sample by
centrifugation; the buffy-coat fraction of the sample is then used to make smears from and since there is
not normally any nucleus or remains of RNA in the mature red blood cell,
staining the DNA/RNA by acridine orange may help to
quicker detect the intracellular parasites, trophozoites and
ringforms, plus also stain DNA/RNA of many other bugs like Borrelia etc. ...
In 1000x magnification it is possible - by turning
the focus handle up and down - to detect if the suspected foreign object is located
intracellular (both the object and the cell membrane stands equally clear at the same
focus level), or if the object is located under or over the cell, where
either the object or the cell membrane stands clear.
With my experience from 10 years of blood microscopy, I can NOT
recommend looking for parastites or microbes at less than 1000x
magnification, nor on conventional thin and thick "malaria" smears! -
is simply not sensitive enough to detect low level parasitaemia! - this
is also supported in the published literature.
For
detecting
borrelia and ringfoms I always use the 100x oil QBC Paralens objective, with
normal light source primarily, and as I have 6x and 10x ocular
sets for
my microscope, I can screen a sample faster in 600x, and
whenever I find something that need a closer look, I shift to 10x
ocular and view the same area in 1000x without moving the object, and
can exchange one of the
oculars for the microcam that connects to my computer via USA, which
allow me to
take pictures or videofilms of moving objects, like the
spirochetes. If I had allowed the sample to stain with
a fluorescent dye like acridin orange or react with microbe specific
FITC-labelled antibodies before the microscopy (remember to
protect the
sample from exposure to light or the fluorescence may fade away, resulting
in a false negative) - I can switch fast and easy between the light and the
fluorescent microscopy modes, by turning off the
normal light and turning on the Paralens external UV light.
Some links on description of immune system / immune cells:
Immune system (WIKI): http://en.wikipedia.org/wiki/Immune_system
Neutrophil granulocyte: http://en.wikipedia.org/wiki/Neutrophil_granulocyte
Toxic granulation: http://en.wikipedia.org/wiki/Toxic_granulation
Monocyte: http://en.wikipedia.org/wiki/Monocyte
Blast crisis: http://en.wikipedia.org/wiki/Blast_crisis
Leukemoid reaction: http://en.wikipedia.org/wiki/Leukemoid_reaction
Microhematocrit technique
description (old desciption using capillary tubes):
http://wps.prenhall.com/wps/media/objects/684/700987/ch07HEMA.pdf
QBC method:
http://www.mdinventions.com/successes/envtests/qmalaria.html
"The
QBC Malaria method is the simplest and most sensitive method for
diagnosing the following diseases.
- Malaria
- Babesiosis
- Trypanosomiasis (Chagas
disease, Sleeping Sickness)
- Filariasis (Elephantiasis,
Loa-Loa)
- Relapsing Fever
(Borreliosis)
Some
research references are shown here.
"
Fluorescence
microscopy equipment (affordable):
ParaLens
(QBC diagnostics) - http://www.qbcdiagnostics.com/products/fm/pla/fab.asp
...
http://www.qbcdiagnostics.com/products/fm/malaria/files/malaria_appnote_pla.pdf
- is a microscope
attachment designed to provide the benefits
of flourescence microscopy to any light microscope.
The old Paralens system which I use (PDF
printout from wayback machine) have a rather
expensive lightbulb of
short duration as the light source, and a fiber optic cable connecting
the
rather heavy external light source to the special QBC objectives, which
comes in both
60x oil and 100x oil, while the new Paralens Advance
(right picture) system (PDF
printout) has a much more
durable LED light source, and is much smaller, light weight, more
handy, and different power source options. The ParaLens Advance
Portability Pack includes options such as a solar battery pack, USB
cord, 9-volt battery clamps and more, while the QBC Mobile Power
Station is designed to power the ParaLens Advance as well as other QBC
products, with a 22 Amp-Hour rechargeable battery and AC/DC
inputs. Thus, the system is ideal for ind the field work!

old versus new Paralens

LUMIN LED fluorescense -
looks like nearly identical QBC Paralens-Advance copy? (who
holds patent?):
http://ledfluorescence.com/index.shtml
http://ledfluorescence.com/fluorescencemicroscopy.shtml
http://ledfluorescence.com/diagnostic.shtml
"Fluorescent
antibody (FA) technique, also known as immunofluorescence, is an
excellent rapid diagnostic method. FA is easily done, sensitive,
specific, and relatively inexpensive. It is extremely versatile. FA
detects and identifies both etiologic agents of disease (direct
FA ~DFA) and host antibodies (Indirect FA, IFA). A wide variety of infectious
diseases can be rapidly diagnosed by FA. Kits and reagents for FA tests
are commercially available, and many use highly specific monoclonal
antibodies." ...
"Commercially available monoclonal antibodies
can be used for doing indirect FA rapid tests to diagnose other
infectious diseases (fluorescent anti-globulins commercially
available). Some of these are: (see table on the website)" ...
"Polyclonal
antibodies to these etiologic agents and others are also commercially
available. Many of these
are suitable for both direct and indirect FA tests."
FITC-labelled Borrelia burgdorferi antibodies can be
purchased commercially from KPL:
http://www.kpl.com/catalog/productdetail.cfm?Catalog_ID=1&Category_ID=144&Product_ID=702
Description: Affinity purified polyclonal
antibody to Borrelia burgdorferi made in Goat and labeled
with fluorescein isothiocyanate (FITC). Isolated from a serum pool of
goats immunized with heat killed whole cells of Borrelia burgdorferi. The antibody is highly
specific for Borrelia burgdorferi. Cross reactivity to
Borrelia hermsii, Borrelia coriaceae, and Borrelia anserina has been
minimzed through extensive affinity adsorption. Product is
in lyophilized form. Each lot is tested to assure specificity and
lot-to-lot consistency using KPL's in-house ELISA assay.
Zeiss - Primo Star iLED
fluorescence microscope:
http://www.zeiss.de/C1256D18002CC306/0/969C99940105AB12C1257489004079FC/$file/60-2-0017_e.pdf
Euroimmun - EURO-Star III plus fluorescence microscope:
http://www.euroimmun.de/index.php?id=produkte_geraete_software&L=1
http://www.euroimmun.de/fileadmin/template/images/pdf/YG_0301_I_UK_A10.pdf
"A
camera can be fitted to the phototube for digital image recording.
Switching between the camera and the eyepieces is unnecessary due to
the convenient 50/50 beam splitter. For the display and management of
digitally recorded fluorescence images we offer the efficient
EURO-Picture programme. The standard EUROStar Ill Plus is equipped with
a halogen lamp for normal transmitted-light microscopy in bright-field
and dark-field and can be upgraded for phase contrast."
SO - in conclusion with
modern equipment using a LED light source
of low energy demand and very long durability, it is no longer
extremely expensive to
get a microscope system that can do fluorescent microscopy plus light /
phase contrast / darkfield.
The challenge is to find a to the microscope suitable
and wellfunctoning microscope CAMERA that
can connect easily to and be powered by any computer via USB!
It must have a fairly high resolution and not the least
must be LIGHT
SENSITIVE ENOUGH
to make it possible to take nice pictures and/or videos as
illustrated. During microscopy the video can run and copy all seen,
snapshots and videoclips from the raw film can be extracted later. This makes it much easier to find microbes, even a very
thin/slim spirochete,
that is either stained orange-red by
Acridine Orange (cheap, see pictures above) or green with FITC-labelled Borrelia
specific
antibodies (see below) ..
- photographing a single green
spirochete up against a very black
background is very camera demanding, I can unfortunately NOT photograph
a slim green spirochetes on a black background with my camera,
despite it is just possible to see such in the microscope!
Affordable microscope cameras
like the DinoLite
series comes in many different
versions; I now use the DinoLite AM4023X for my old Leitz Laborlux III
microscope anno 1957, because I do not have the trinocular top for
it; but - despite it has a
higher resolution of 3 Mpixel - it is unfortunately not quite as light
sensible as my old Bresser Microocular II camera (pic),
the very big problem
with my old Bresser microcamera is that is has only VGA resolution
(640x480
pixels) and worse, there are only 32-bit drivers for this
32-bit device, i.e. no drivers can make it work on a 64-bit
Windows (Vista-64 or 7) system, therefore I had to buy a new camera after shifting to a Windows7 computer system!
Plus of course one need good software i.e. a picture and
video grapper (can be found as free download) and editor
program
(free Windows Movie maker is okay, but lack marking tools and can only
save video in WMV format) - one need at program that make it
possible to measure and mark structures, can put on arrows, rings, and
text remarks on both still pictures and video etc. The DinoLite
software works very well, but the program only works with DinoLite
cameras of any type.
Note also the hand or stand held
DinoLite microscopes which magnification ranges from ~10 to
200 to 500x, depending on
the version; LED lights
are built in in white, polarized, ultraviolet, infrared or a switchable
combination. Most versions have calibration and measuring options. Its
housing is either composite or aluminium alloy ...
Very nice "toys" that can be used for many purposes at affordable price, like photographing ticks
... what will future bring of enhancements?
Borrelia:
Borrelia burgdorferi specific direct fluorescent (FITC) immune stain:
 Picture taken by Alan MacDonald (ca. 1985), borrowed with
permission.
Picture taken by Alan MacDonald (ca. 1985), borrowed with
permission.
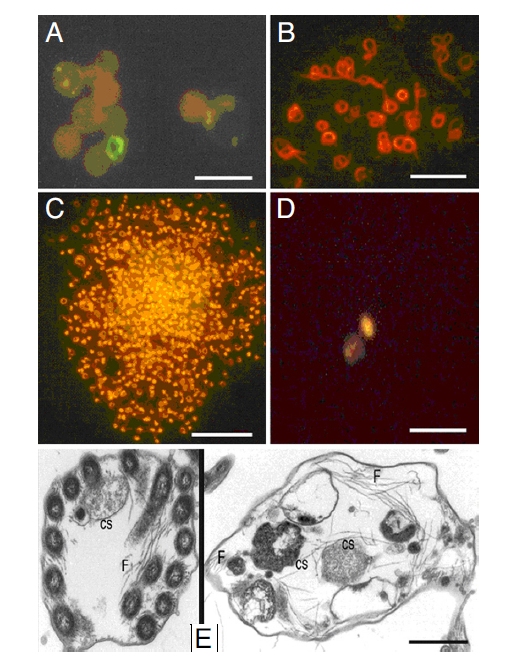
Both spirochete and cyst form and "granules" stain with
Borrelia antibodies!
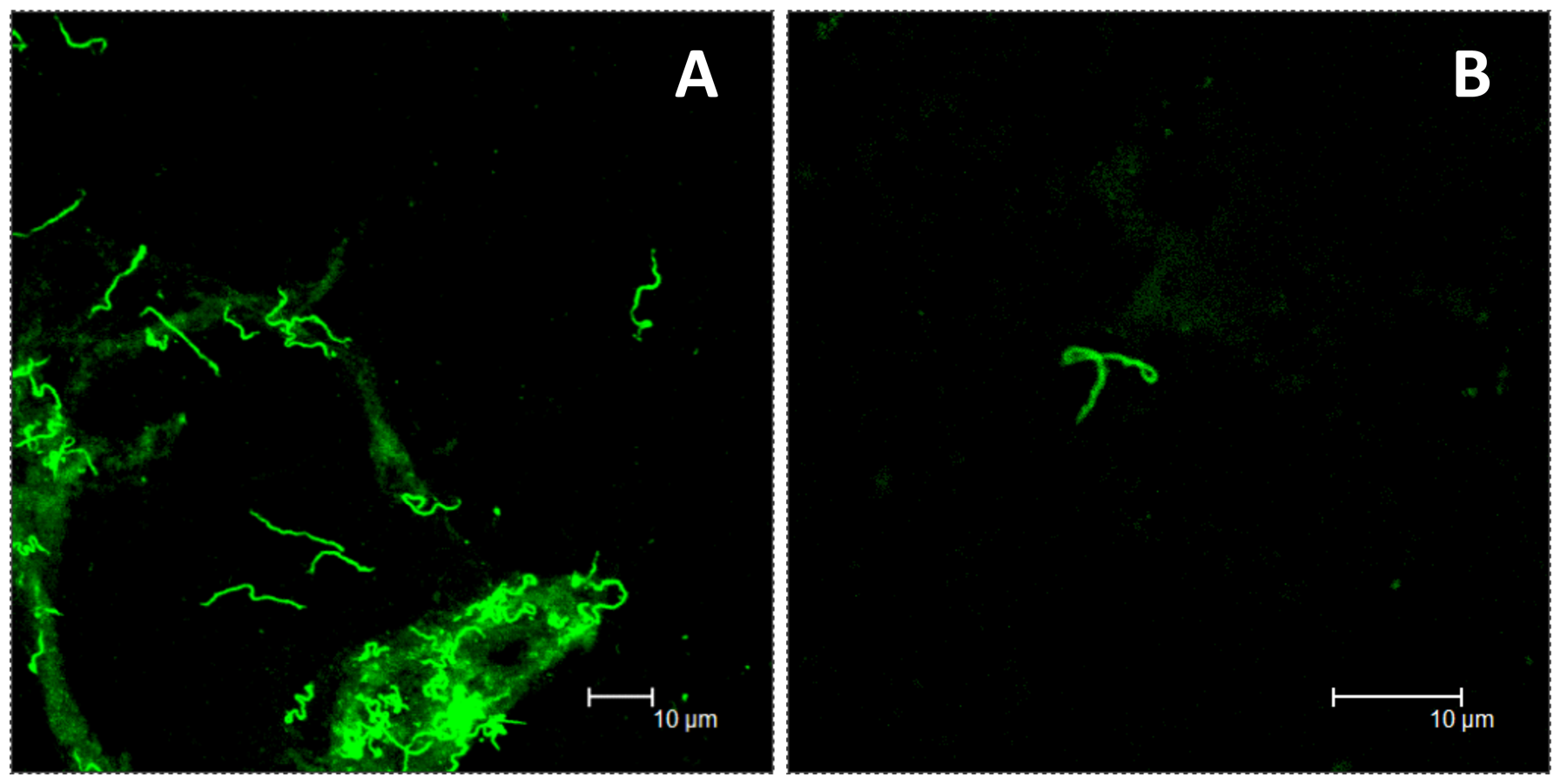 From Embers et al. (Full text):
From Embers et al. (Full text):
Persistence of Borrelia burgdorferi in Rhesus Macaques following
Antibiotic Treatment of Disseminated Infection (2012)
Figure
5. Spirochetes
recovered by xenodiagnosis from animals treated in the disseminated
phase of infection.
Images
from direct fluorescent staining of B. burgdorferi
spirochetes found in xenodiagnostic tick midgut culture (A)
or tick midgut preparation (B) from treated animals GA59 and GB56,
respectively.
Note that the above
Borrelia
burgdorferi spirochetes do not look very spiralled
(In TBRF pictures the spirochetes often seem more coiled than B. burgdorferi).
BUT all those who claim that
Borrelia burgdorferi spirochetes has so and so many coils
and is always of a certain length and thickness
- and say that
what I call spirochetes do NOT look like ideal Borrelia spirochetes from pictures
- have obviously not spend years studying the morphology and
movements of live spirochetes in the microscope themselves!
Enjoy especially the amazing movies at
(Youtube searching for Lymebugs videos) - all microscopies were made by Stan Dembowsky
in 1999 from his studies
of the laboratory grown spirochete
Borrelia burgdorferi B31
... demonstrate:
Borrelia uncoiling itself and changing morphology at its will pretty fast
Growth by division, growth in colonies within biofilm like substance,
adults spirochete form and baby-spirochetes, blebbing, cyst form ..
Growth of Borrelia B31 on sheep blood agar, where baby-spirochetes
seem to be emerging from the surface of the red blood cells?
Stan also studied the effect of penicillin on Borrelia B31
...
CDC on Tickborne
relapsing fever borreliosis (TBRF): http://www.cdc.gov/relapsing-fever/clinicians
The very important Borrelia
relapse pattern:
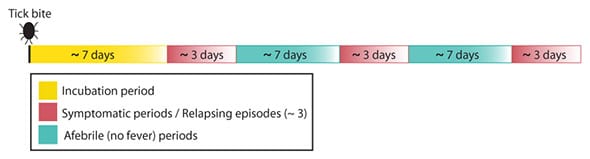
Note that spirochetes are only visible in
the blood during the symptomatic episodes, but the blood was still
(more) contageous also in between the relapses, when there is no
visible
spirochetes in the blood: http://lymerick.net/1914-Nicolle.htm
=> CORRECT TIMING of sampling of blood specimen for microscopķc
examination for spirochetes in relation to the patient relapse cycle
(during attacks) is essential, if one does not time the
sample
right there is a high risk of not seeing any spirochaetes!
In
case the patient has a cycle that runs over 10 days, we only have
10% chance of doing it on day one in a new relapse, if one just take a
random sample, and NOT time the sampling according to the relapse
cycle; in case a patient has a monthly relapse pattern the chance of
finding spirochetes is 1/30 i.e. 3%!
I only have greater
"luck" finding spirochetes by microscopy, than most other
investigators, because I understand the relapsing pattern, and plan
after it; I ask the patients to do a very detailed
Excel symptom diary
(OA but respect my ownership, dont remove my name) and from the relapse
cycle I can deduct, when is the best time to see the patient and
do sampling for direct test for spirochetes! - and can judge effect of
any intervention.
A immunocompetent
relapsing fever Borrelia
patient will typically get from 10-30 relapses, before the disease
"burn out" when the immune system achieves control over the microbial
proliferation .. probably likewise for most patients infected with Borrelia burgdorferi?
Symptom flares (attacks) are
gradually
diminishing in severity (relating to the amount
of spirochetes that enters the blood and raise complement
cascade reaction (you should measure complement split products if available) and proinflammatory
cytokine storm (TNF => fever, IL-1, IL-6 ...) and late in the
course, also the intervals between
relapses is increasing, as long as the
infection is kept under control (by immune system and/or antibiotics) then
newly
formed spirochetes are not allowed to reproduce
themselves weekly any more ...
It is usually
not mentioned in the literature that Lyme borreliosis also clinically gives a relapsing infection pattern, just like
the close relatives in the relapsing fever Borreliosis group
(except TBRF is perhaps more pathogenic, cause a higher TNF
(fever) response than the Lyme borreliae
does in the immunocompetent host?).
BUT there are some works examining this subject ..
In very ACTIVE
LATE (> 6 months post infection) Lyme
borreliosis, where moving spirochetes can repeatedly be found in
buffy-coat blood fraction from the patient by microscopy, but usually
only during the first day of a new flare, the clinical relapse
pattern is the
same, weekly relapse pattern, as descibed in TBRF!
Whenever the borrelia infection is under relative control by the
immune system and/or antibiotic treatment, the relapse cycle shift
pattern, usually within 4-6 weeks after start on antibiotic
treatment, from
the previous weekly to a monthly cycle at 3-4 weeks intervals!
The
monthly clinical relapse
cycle was described by dr. Joe
Burrascano in his diagnostic guidelines, already since the
early 1990ies, see ILADS:
http://ilads.org/lyme_disease/treatment_guidelines.htmlCyclicity was also noted by Willy Burgdorfer ...
Lyme borreliosis: a
relapsing fever-like disease? (Burgdorfer 1991)
http://www.ncbi.nlm.nih.gov/pubmed/1947807
...
[for up to 3 months after animal aquired the
Borrelia infection
feeding] ticks were evaluated for spirochetal infections by direct immunofluorescence.
All mice were found to circulate spirochetes for at least three months
in concentrations sufficient to infect ticks. The percentage of infected ticks
alternated from low to high, suggesting occurrence of episodes of mild
and heavy spirochetemias. The
results suggest that B. burgdorferi in its animal hosts and possibly
also in humans causes prolonged spirochetemias characterized by
episodes of alternating high and low concentrations of spirochetes as
reflected by similar percentages of infected ticks.
The long persistence of spirochetes in the peripheral blood stream and
the cyclical
form of Lyme borreliosis appear to be related, as in
relapsing fevers, to the capacity of B. burgdorferi to undergo antigenic variations."
The two relapse patterns, that are both characteristic of clinical Lyme
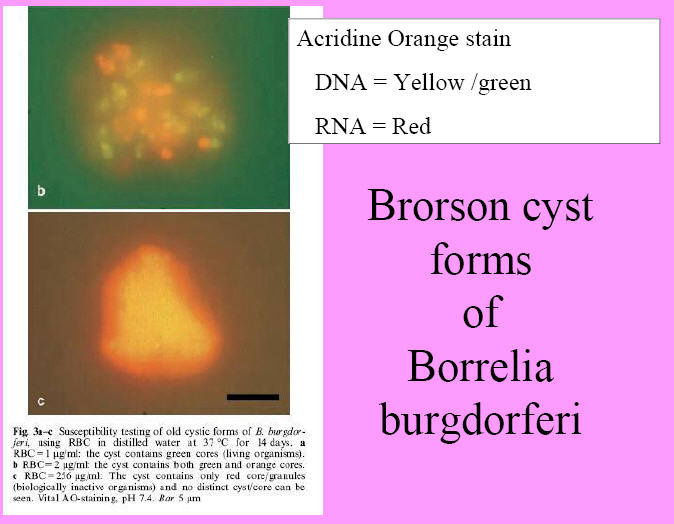
borreliosis, are probably well explained by Brorsons laboratory
culture studies, finding that it take the
YOUNG cystic forms of Borrelia
about 9 days versus for
OLD cystic
forms about 4 weeks to
reproduce the spirochaete form:
http://lymerick.net/1998-Brorson.htm
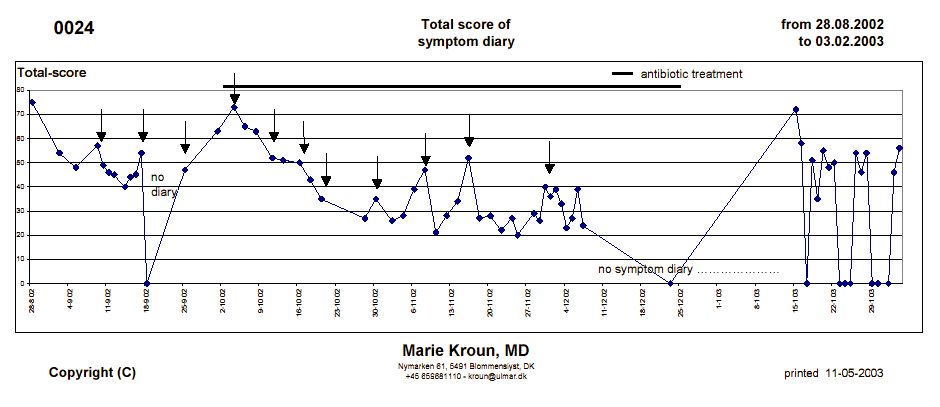
The patients with VERY ACTIVE, weekly,
RELAPSE pattern usually will respond to
antibiotic treatment in a quite typical and gradual way, as illustrated
by symptom log curves from case#24, who kept my Excel symptom diary
(early version); there are 7
days in between the dates on the X-axis; arrows indicate the
circa
weekly
clinical worsenings (6-14 days acc. to old research on RF-spirochete
lifecycle done a century ago):
The
monthly relapse cycle
(some more intensive than others) is illustrated by curve from
project patient #11 (still one week in between dates on X-axis):

More on videomicroscopy for spirochetes and the Excel symptomdiary
(free download):
http://lymerick.net/MK-videomicroscopy.html
My studies done before 2006 using direct fluorescent immunostain with
borrelia specific antibodies from KPL (done as research in BRT),
and primitive cheap
equipment:
http://lymerick.net/videomicroscopy.htm
Other reports on chronic (late diagnosed) or persistent (post
antibiotic treatment relapse) borreliosis
documented by positive culture,
microscopy and/or PCR:
http://lymerick.net/persistent-borreliosis.htm
Borrelia culture: http://lymerick.net/Borrelia-culture.html
Culture in
commercially available BSK-H medium* is often
successfull, especially when there are a few visible spirochetes in the
wet drop
sample from a fresh buffy-coat prep.; after positive culture much more
material can be examined by other methods, i.e. there is a possibility
of PCR typing of the Borrelia strain when there is enough spirochetes.
DON'T THROW MATERIAL OUT AFTER ONE MONTH as some researchers do,
according to published reports on culture. In adverse conditions it may
take much longer for slow Borrelia to grow in
vitro:
http://lymerick.net/Borrelia-growth-optimum.html
*) Sigma-Aldrich http://www.sigmaaldrich.com/catalog/product/sigma/b3528?lang=en®ion=DK
I
read (but forgot in which ref.?) that some found that
Borrelia cyst may not grow spirochetes well, unless they have
experienced a colder period, like they would have experienced
in a
natural life situation, when residing in dormancy in a tick
during
the winter-time. Can the microbes sense the seasons somehow?
Many
chronic patients (that are not in antibiotic treatment), descibe
getting longer worse periods, shifting to a weekly relapse pattern
during spring-time (late march-mid june) and during autumn (mid-august
to late november), while they shift to a monthly relapse cycle during
high summer (high temperature, low moisture so ticks are not
questing), and monthly cycle during winter time ...
- can tickborne
microbes sense somehow the seasons when ticks are active questing for
blood meal out in the nature, and become active then, while they go
into dormancy at times when the chance of a tick biting it host is
lower? Please
note, that all microbes that use the TICK - with ½-1 year between blood
meals - as vector, ALL have the ability to go dormant in the tick for
at least six to twelve months, otherwise they would not be able to keep
the infectious tick-mammal-tick life cycle going. The microbes also go
dormant inside their mammal host, when the environment becomes
unsuitable for the spirochete form, like when the patient forms lots of
antibodies, under antibiotic treatment, lack of nutrition, wrong pH,
too high oxygen etc; persisters can survive common treatment and some
cnb awaken later when the environment again favours regrowth of
spirochetes, that has been dormant for a very long period.
This was observed by Hampp already before 1950! -
http://lymerick.net/1948-Hampp.htm "Typical free granules, the end products of granule 'shedding', are shown in
figure 18.
They are roughly circular in
outline and sharply bounded. They consist for the most part of what appear to
be short sections of spirochetes closely packed together. The
contents of these granules are probably responsible for the fine lacelike appearance
and the bright white, highly refractile bodies
described by Hampp (1946) under the dark-field
microscope.
Examples of another type of free granule repeatedly observed are shown in
figures 19 and 20. These granules consist of tangled masses of spirochetes or spirochetal segments.
The significance of granules in the
life history of the spirochetes is unknown but certain investigators have
suggested that they may be germinative units (Balfour,
1911; Noguchi, 1911; Noguchi, 1917; Leishman, 1918; Mudd et al., 1943; Hampp, 1946).
Others are undecided or hesitant in accepting this hypothesis (Fantham, 1916; Akatsu, 1917; Wenyon, 1926; Warthin and Olsen,
1930). Topley and Wilson (1936) have indicated that
they are probably particles of culture medium adhering to the sides of the
spirochetes. The electron micrographs demonstrate that this explanation is
wrong, and that free granules
are definitely a phase in the development of spirochetes. Although
it is not possible to determine from these micrographs that the granules are germinative units their constant rhythmic occurrence in
living cultures suggests this possibility. Further support of this hypothesis is provided by the fact
that cultures up to 31 months old, showing only refractile
granules by dark-field examination have invariably given normal growths on
transfer to fresh medium (Hampp, 1946)"
31 months is circa 2 ½ years!
DualDur reagent, Dr. Bozsik patent
description (2004) - double centrifugation technique
(removes blood cells):
http://www.patentgenius.com/patent/6689577.html
"In our
experience, it is still possible to detect the pathogenic
bacteria if there are less
than 10 bacteria in a milliliter of
centrifuged native blood samples. In comparison, the
threshold for the
detection of Lyme borreliosis with PCR, which is currently considered
the most sensitive but can only be done in specially equipped
laboratories, is between 40 and 100 germs per ml; besides, as many as
possible primers specific to different sub-strains should be available."
[20
drops per ml, i.e. 1 drop is 50 microliter, i.e. there may be one
spirochete per 2 drops of HIGHLY CONCENTRATED BLOOD sample, after
double centrifugation! .. of course one will need to examine more drops
in order to find the spirochete, if there are so few - the investigator
must be very meticulous!]
Detection of Borrelia in
acridine orange-stained blood smears by fluorescence microscopy.
(1983)
http://www.ncbi.nlm.nih.gov/pubmed/6602602
"We concluded
that the AO stain is simple, rapid and more sensitive than Romanowsky
methods for detecting cases of low-level spirochetemia."
SHORT REPORT: Detection
of Borrelia (Relapsing fever) in rural Ethiopia by
means of the quantitative Buffy Coat Technique.
(2001)
http://www.ajtmh.org/content/65/2/164.full.pdf
"In
laboratory studies that used Borrelia burgdorferi as a model, we detected spirochetes at
concentrations as low as 10 organisms/mm3, whereas the
number of positive readings assessed by means of stained blood films
fell significantly at dilutions below 3,263 organisms/mm3. The greater sensitivity of the QBC
technique is important in areas where Borrelia is endemic.
.."
Note
10 / mm3 = 10 / microliter, i.e. correspond to 500
organisms per examined blood drop; number of spirochetes
in RF is much, much higher than in Lyme borreliosis, where
> 7 organisms per drop is "many"!
Babesia:
Phylogeny and evolution
of the Piroplasmida as inferred from 18S rRNA sequences.
(2012)
http://www.ncbi.nlm.nih.gov/pubmed/22429769
The order Piroplasmida consists of several genera of
tick-borne parasites that infect mammals, and to a lesser extent birds,
and are therefore of medical and economic importance. Despite their
importance, considerable confusion exists concerning the relationship
among piroplasmid species, specifically concerning the number of genera
and the intergeneric relationships.
To examine evolutionary
relationships among piroplasmids, we conducted phylogenetic analyses of
192 18S rDNA sequences from the genera Theileria, Babesia and Cytauxzoon.
Our analyses revealed eight
clades potentially representing distinct genera, and we distinguish the Duncani Group and Microti Group as
genetically distinct groups of species requiring detailed analysis of
morphology and life-history to allow formal generic description.
The piroplasmid phylogeny
revealed considerable host diversity and limited host specificity,
suggesting piroplasmids have undergone frequent host switches during
their evolution. Our analyses provide the first reported evolutionary
timescale for piroplasmids independent of the assumption of
parasite-host cospeciation, which is invalid for piroplasmids.
Evolutionary rate analyses revealed considerable substitution rate
heterogeneity, which we attribute to host switching and
diversification. Finally, we call for a comprehensive phylogenetic,
morphological and life-history analysis for these medically relevant
taxa to resolve relationships and understand host specificity.
The application of
acridine orange staining to quantitate low levels of Babesia divergens
parasitaemias. (1974)
"Our cases highlight that, in
Europe, babesiosis can occur in healthy persons and manifest as
moderate illness.
The rarity of other reported cases in nonimmunocompromised patients in
Europe may be related to the difficulty of diagnosing babesiosis. A
stained thin blood smear is rarely performed in
Europe after tick bite in healthy patients. The difficulty of detecting
intra-erythrocytic forms of babesia coupled with frequent low levels of
parasitemia, may result in accurate diagnoses, although acridine orange
and fluorescent microscopy may assist in the detection of parasites
(1). Other diagnostic tests, such as PCR and serologic
analysis, are
not routinely performed in France and require a reference laboratory
(8). ....
Babesiosis,
although difficult to
diagnose, needs to be diagnosed for various reasons:
1) without
treatment, babesiosis can lead to severe illness;
2) the disease can
persist for a long period without symptoms, which could lead to
posttransfusion cases (12); and
3) effective specific treatments are
available (atovaquone plus azithromycin, or for severe cases,
clindamycin and quinine) (2).
These drugs are not usually prescribed in
febrile tick-bite cases; doxycycline is the usual drug used to treat
tick-borne bacterial diseases. Moreover, patients with moderate
infection could benefit from an atovaquone plus azithromycin
regimen, which is better tolerated (13). ...
In
Europe, babesiosis is probably underdiagnosed; thus, we suggest that
when patients have influenza-like or malaria-like syndromes after
confirmed or suspected tick bites, a blood smear be performed
regardless of whether the patient is immunocompromised. Blood smear can
identify not only Babesia spp. infection but also Anaplasma spp.
infection, another emerging and underdiagnosed tick-borne
illness. In
cases of new European Babesia spp. infections, a deeper
characterization of the strains by erythrocytes cultures and
standardized PCR, as well as a systematic study of the patients’ immune
systems, should be undertaken to enable a better understanding of this
disease."
Babesiosis:
recent insights into an ancient disease. (2008)
http://www.ncbi.nlm.nih.gov/pubmed/18440005
"Most
significantly, molecular
analysis of the implicated pathogens suggests that the host-range of
many babesia is less restricted than believed previously
hitherto unrecognised species can cause infections in a variety of
animal hosts and in humans (Zahler et al., 2000: Cho et al., 2002:
Herwaldt et al., 2003, 2004; Conrad et al., 2006: Kjemtrup et al.,
2006: Häselbarth et al., 2007: Kim et al., 2007). Therefore,
many past cases of human habesiosis on both sides ot the Atlantic that
were attributed, hased on traditional methods, to classic species such
as B.
divergens or
B.
microti, may
indeed be due to species not yet known to cause such infections in
humans
(Herwaldt et al., 2003: Gray, 2006: Hildebrandt et al., in press).
This
notion is further substantiated by the recent recognition of Babesia
duncani [WA1] and B. divergens-like organisms as pathogens of
medical significance for humans in the US (Herwaldt et al., 1996;
Beattie et al., 2002: Conrad et al., 2006). Moreoven, confirmed autochthonous (~local
strains of) B. microti infections have been reported in Taiwan, Japan
and Europe (Shih et al., 1997: Saito-Ito et al., 2000:
Hildebrandt et al., 2007), and a
new European B. divergens-like organism (EU 1), provisionally named
Babesia venatorum, has been discovered,
which is probably a parasite of deer (Telford and Goethert,
2004:
Bonnet et al. 2007). This parasite was involved in the first documented
cases of human babesiosis in Italy, Austria and Germany ( Herwaldt et
al., 2003; Häselharth et al., 2007). ..."
SUMMARY:
ALL THE OLD ASSUMPTIONS HAVE PROBABLY BEEN WRONG
- since during the last 2 decennials many new types of microbes have
been detected after PCR typing began - and genera shows greater
diversity and little host specificity within the Babesias, as well as
in the other tick transmitted infections!
Humans with ringforms in their red blood cells, appears to have not yet been investigated for if they instead of Babesia, could be infected with the closely related other piroplasmida, that infect a variety of animal hosts, Theileria and Cytauxzoon!
Note: It has been proposed by some to move/rename Babesia microti to Theileria microti, based on a closer genetic relationship of this group to Theileria, than to other Babesia spp.
Theileria infect both lymphocytes and red blood cells, and can form multinucleate intralymphocytic schizonts. More about Theileria in this PDF.
Below focus
is set mainly on two of the newly found Babesia types, first
called EU1 and WA1, because they seem esp. relevant to DANISH patients:
1. Babesia
duncani (former WA1):
Anti-WA1
antibodies were detected in a high fraction, no less than 14 of 132 (10,6%)
danish
neuroborreliosis patients, had antibodies reacting with Babesia WA1,
an - at the time - very newly in the USA found Babesia variant, acc. to
conf. abstract presented in USA 1996.
Unfortunately no further danish studies of Babesia were done, acc.
to the lead author Anne-Mette Lebech, they put the work away, thinking it was "all false positives"! ...
There
is no smoke without a fire!
- if we do not have Babesia duncani in Europe,
we do have other Babesia gibsoni-like spp. here, that might
infect
people and may give rise to antibodies that crossreact with
the
Babesia gibsoni-like B. duncani antigen?! .. it would be prudent to
search for which infection could have raised cross-reacting
antobodies!
This should
immediately have been properly investigated of course, in
ticks,
in reservoir animals and in sick humans that did not recover after
conventional antibiotic treatment for Borrelia - but sick people get ignored!
MANY, including
myself, have been told "we dont have Babesia
here in Denmark, so there is no reason to test you for it" - but when
we got tested, many had ringforms inside red blood cells, besides
borrelia!
In 1998 I asked for
test for co-infections, including Babesia, Ehrlichia, but I was
denied testing, reason being told "not available in Denmark, and too
expensive on the departments budget to send tests abroad"!
I was very sick and
soon after had to go on permanent sick leave / early retirement; not
getting proper diagnostic help from my danish colleagues, I
searched the world for other test options (paid out of own pocket) and
was in 2001
diagnosed in USA with certain persistent borreliosis by direct immune
stain for Borrelia burgdorferi, very likely babesiosis, since
there were ringforms in several of my RBCs, and possible MONOCYTIC
Ehrlichia; then I ordered serologies, but Statens Serum Institut
(SSI) decided not to send
my samples abroad for testing with serology nor PCR for
the co-infections, despite
there were published reports showing it could have been done; the
local microbiology department did (very surprising to me) not ask for
serial
bloodsmears, so I had to do it all myself. Got the serial
smears stained by the local pathology lab. who mounted coverglass
on the smear - so later these many serial thin
bloodsmears were reexamined
by Italian vet. Walter Tarello. The comparison result is reported in
my York 2004
presentation. Unfortunately I did not get the smears back :(
SSI could and should have
researched
PubMed via Internet. They could have found and read about the
method used by the Japanese for detecting babesia i farm animals, that
was used in 1999 in the first human Japanese donor transmitted
case investigation - http://lymerick.net/York2004/Japanese-donor-Babesia.htm
- which serves us now, as example of very good clinical and research practice!
The
Japanese investigators had traditionally investigated bovine babesiosis
cases by inoculating blood from sick babesia suspected cows into special breed of immune
deficient laboratory mice, whose blood had been exchanged with blood
from healthy cows and likewise for other animals; the blood
exchange
procedure creates a much more Babesia sensitive host animal, at test system that can detect
infection in very low-level carrier state, below detection with
microscopy and PCR, like in healthy blood donors; they copied the babesia
detection method from animals, except for using human blood instead, i.e. the
NOD/shi-scid
mice circulating erythrocytes (RBCs) had been replaced with
human RBCs
(hu-RBC-SCID mice) to facilitate "culture" of the parasites in the mice.
NOTE: The donor's serum
exhibited
a high antibody titer against the local Babesia microti-like isolate
from the patient, whereas it
exhibited only a weak cross-reaction against (three) B. microti strains
isolated in the
United States.
"Examination
by microscopy and PCR failed to detect the parasite in the donor's
blood
obtained 8 months after the donation of the blood that was transfused.
However, we were able to isolate Babesia parasites by inoculating the
blood sample into SCID mice whose circulating red blood cells (RBCs)
had
been replaced with human RBCs."
LESSONs learned:
1.
asymptomatic donors can have so low level carrier status of
Babesia, that it can not be detected by microscopy, nor by PCR, but
still there are enough parasites to pose a risk of transferring
infection to susceptible blood recipients via transfusion! - the least
one can do is to ask donor about risk factors for aquiring Babesia
infection, which is not done routinely by the blood banks!
2.
the Japanese (Kobe) Babesia microti-like strain could be detected
by US B. microti PCR assay, while the antibody response measured
very weak with 3 american B. microti strains as antigens; both patient
and
donor had high antibody titers against the local variant => the same
situation might apply in Europe vs USA, i.e. when danish SSI lab.
send samples to CDC - that are using US
Babesia strains as test antigens? - these test may not detect
the European patients infected with local European variants,
who may test very
low or false seronegative, due to strain antigen variation.
The best
must be to try to isolate and culture local strains and then use these
strains as antigens in indirect IFA assay, exactly what the smart Japanese investigators did!
If the highly
susceptible hu-RBC-SCID mice,
after inoculation of a suspects blood, develop a very high
level of
parasitaemia, the
blood of the mice can be used in homologe IFA serology test,
with the patients and the donors serum. Local IFA i.e. with locally found antigens / strains can be developed;
once the local parasites are catched from patients or local
reservoir animals, it should be reasonably easy to maintain the local
strains in animal culture in abundance enough to do many IFA tests
by copying the Japanese method, using hu-RBC-SCID
mice; how
to develop a local IFA is explained in detail in the Japanese article in english!
In
USA Babesia duncani (WA1) infection seem to be a rare*, but more
serious - also in previously immunocompetent individuals that have
not been splenectomized - compared to Babesia microti that is
often described as mild (by doctors, patients tell different) and
rarely fatal (mortality rate can only be calculated for the
diagnosed cases, there may be many undiagnosed cases).
*) VERY IMPORTANT TO
KNOW is that Babesia duncani (WA1) parasite is genetically distant from and
is not
crossreacting with B. microti, so Babesia duncani
infection is not found by PCR
and antibody test for B. microti which is normally the
only Babesia test
applied in USA! - see references below!
*) rarely diagnosed can be because the right test is not done at the
right time in the patients disease course, or because of testing for too different strains from the on infecting the patient!
Strain variation make commercial serology tests that are based on a single strain unreliable, i.e.
high risk of locally infected patient only showing borderline
positive or false negative antibody result, if a commercial antibody
kit is used with foreign strains from distant areas, that are too
different in antigenic expression from the local variants.
2. While occurrence of Babesia infection in
European ticks is generally found low, with current PCR tests (~1% of
ticks), the
occurrence of Babesia venatorum (EU1) seems to be relatively far more
common in the european tick populations than is B. divergens and other
Babesia spp., according to very recently (2010-2012 published)
investigations from several European countries, references
listed below ..
3. passerine
bird routes up to Norway may fly over Denmark and some of
them was found to harbour Babesia venatorum (EU1), but not other
babesia spp.
The birds may stop for fouraging and resting many times underways,
dropping of or aquiring infected ticks locally, i.e. danish humans and
animals are likely at some (low) risk of getting infected with
Babesia EU1!
=> therefore danish patients suspect of Babesia
infection (ranging from having episodic (4-5 days interval it seem)
mild hemoglobinuria (stix can be done by patient at home, and do not
cost much) and symptom flares, to overt babesiosis i.e. severe
hemolysis resulting in blood-pis, and development of anaemia ... should
first of all be investigated for this most likely Babesia type to
become infected with ...
Unfortunately
none of the previously reported tick studies from
Denmark, examined danish ticks for presence of Babesia spp.,
so we DON'T KNOW
THE FACTS about prevalence of Babesia tick infection rate investigated
in local Danish ticks
- therefore
I needed to estimate the risk of getting infected with different
Babesia species from other studies done in our neighbour countries in
Europa, where the passerine birds may have come from, that later
reach Norway via route over Denmark.
Low
level carrier infections pose a threat because Babesia and other
microbes can be transmitted to very susceptible patients
needing
blood transfusion! - but do not get diagnosed due to lack of tests, to
lack of knowledge / awareness in doctors and the public, or worse by
ignorant and arrogant doctors that should have studied the literature
to know how the best standard (Japanese case study as teacher), but who
chose doing the wrong tests, with too much differing antigens, too low
sensibility, test with an - a priori - very low chance of showing any
positive results!
Donors are not screened for possible
Babesia or other tickborne infections, the blood banks just ask if the
donor feel healthy, then okay to tapping; they do not even ask
if
the donor have had tickbite, known borrelia infection etc.
(asymptomatic long term carrier state of babesia is very well
documented in published literature) ... I NEED TO DO MY PART TO CHANGE
THAT, BY INFORMING AND RAISING AWARENESS!
This
little "review" was prompted by a very recent investigation of
a
danish chronically ill borreliosis patient (rheumatologic "polymyalgia
rheumatica" and started corticosteroid treatment, that were later
diagnosed
with repeated
episodes of hemoglobninuria on stix
-
the patient had a weak /
borderline seropositive (1:128) in a Babesia
serology test, where the test antigen used was not revealed in the report, but it
was probably a European B.
divergens assay (since that is what is usually tested for in Europe)?
- i.e. possibly it could perhaps be a weak
sero-cross-reaction
with the Babesia divergens-like Babesia venatorum (EU1) if the patient has this variant?
.... it would probably NOT give any
cross reaction, if the patient was infected with the Babesia
gibsoni-like B. duncani / WA1, who is more distant from B. divergens ..
Babesia venatorum (EU1) is
probably the most likely Babesia species for humans and animals to
become infected
with here in Europe, judged from a higher occurrence of
this strain in the
European ticks, than B. divergens and B. microti, reports coming from several areas of
Europe - all areas where Babesia has been
investigated so far - this is illustrated in
selected articles below on Babesia venatorum (EU1) strain!
Weeks
later the patients serum was send to CDC, who applied an B.
microti
assay (probably using a US B. microti antigen variant, which can be
problematic acc. to Japanese donor transmitted case, if the
patient has a local Babesia microti-like variant, plus an experimental B.
divergens assay .. relevant question is, did CDC use USA variant of B. divergens, found
in Washington, which may differ from EU B. divergens?) OR did CDC use
a European B. divergens variant, that could possibly show borderline
positive cross reaction, if the patient is truly infected with B. venatorum? ... both
CDC assays came out negative more times ..
Thereafter
the danish microbiology reference laboratory, Statens Serum Institut
(SSI),
recalled the previous seropositive as probably having been a false positive serology,
which they can
NOT, really!
I detected nice ringforms in this
patient blood
(ON43) blood on Nov. 9, 2011, by microscopy of buffy-coat smears
plus
I also found moving spirochete-like structures in wet drop prep. from
buffy-coat fraction, see video (more pictures of ringforms therein too.
The written
report in danish (with pictures and link to video) was immediately send
to the ID doctors in charge of the patients care at the time, so I did
my part to help the patient get diagnosed properly (I
am just a consultant for a danish patient org. with special interest in
direct diagnosis of tickborne infections, doing a little bit of research on my own)!
Written report in danish: http://case.ulmarweb.dk/ON43/ON43-20111109.pdf
Video: http://case.ulmarweb.dk/ON43/ON43-20111109.wmv
BUT
THE MICROSCOPY FINDING WAS APPARENTLY IGNORED! - it is not even mentioned in the
University
hospital infectious disease doctors papers on the case, they don't
count in my microscopy finding in their conclusion on this patients
case, whom they later
dismissed as "never had babesia", based on neg. serology and PCRs that
could have been false negative, if not using the right strains as test
antigens / using wrong primers for PCR!
-- the patient was offered no treatment
neither for ringforms nor spirochetes, except CORTICOSTEROIDS, a
perhaps dangerous treatment for the patient!
None
of them answer the patients and my good question: which other
Babesia-like antigen could then have produced a false positive
serology in the patients?
Furthermore,
the very same day I did extremely thorough blood microscopies on
buffy-coat fraction from the patient and found ringforms in
more
of his buffy-coat smears, the ID department in
the University hospital send
this patients PLASMA for Babesia PCR
(unknown primer), and with negative outcome, OF COUSE! ...
since a
positive outcome of PCR could not be expected,
because
Babesia parasites resides inside the red blood cells, which are NOT
PRESENT in the PLASMA FRACTION they send for PCR !!!!
The doctors
responsible for care of this patient chose
to conclude that this patient never had babesia infection!
... which they can
NOT, really, based on the investigation they have done,
because they have not done specific test for Babesia
duncani (WA1) nor for Babesia venatorum (EU1)! ...
False negative PCR test
results can be a result of very low degree of parasitaemia
(like in the Japanse donor 8 months post infection of a blood
recipient) and perhaps the degree of parasitaemia may fluctuate over time,
it is probably way under 1%, since it can not be detected on
conventional
thin and thick smears, nor by PCR AND/OR due to use of the wrong
test, i.e. they only tested for Babesia microti and Babesia divergens
(both
USA variants?), and used negative results to dismiss the previous borderline positive Babesia titer.
My
guess is, that this patient could have a Babesia venatorum EU1
infection cross-reacting minimally with B. divergens as test antigen?
NEITHER
SSI (nor CDC) HAVE BOTHERED TO INFORM US IN THEIR TEST REPORTS
EXACTLY WHICH BABESIA ANTIGENS AND PCR PRIMER THEY USED IN THE
NEGATIVE TEST!
NECESSARY INFORMATION IN ORDER TO REMOVE ANY
"REASONABLE DOUBT" THAT THE RESULTS COULD ALL BE FALSE NEGATIVE,
BECAUSE THEY CHOSE WRONG STRAINS WITH LOW RESEMBLANCE TO LOCAL
STRAINS!
This patients
(and all other suspected) Babesia case(s) should be examined just as
thorough as the Japanese index case was investigated 10 years ago, i.e.
by inoculating the patients blood on SCID-hu-RBC
mice, and if
parasites can be cultured this way, serology must be done via IFA using
the patients own strain as the test antigen, just like was done in the Japanese case!
Many doctors have
told danish patients "we dont have it here, so there is no need to test
you for it" and have dismissed "borderline" serology results as false
positives ...
There is no smoke without fire! .. a borderline
means something that need further investigation, not to be dismissed as
if it could not be of any causal significance, especially not if the
patient is chronically il, without any other reasonable explanation
found for the chronic illness - it would be prudent to investigate
properly - thinking "out of the box" on uncommon or even new
infections, when the patient present like this one, with repeated
episodes of hemoglobinuria, and concurrent fever and elevate CRP!
The implication of we now have recognised that passerinee
birds may carry ticks over several thousands of kilometers from Africa to Norway,
that
may be infected with multiple human pathogenic microbe
species,
that may be transmitted to pets and people along routes of the flights and CAN make the animals very sick
-
explains why neither BORDERS nor FENCES can contain infections
and
prevent the spread; if we don't have it here already, it is very
likely just a matter of time before we get it ... "WE DON'T
HAVE IT HERE" is probably a very wrong statement! ... we do have it, but need to do the right tests to find it!
PROPER
CASE INVESTIGATION AND COMMANDED SURVEILLANCE OF OCCURRENCE OF ALL THE
TICKBORNE INFECTIONS IS NECESSARY, BETTER LATE THAN NEVER!
- using the very most sensitive diagnostic methods of course!
4. Personally I am quite
sure we already have more Babesia spp. here in Denmark already
(vets recognize that cows get Babesiosis sometimes), and
probably
more types of Babesia, just like in our neighbour countries ...
I,
personally, was the very first human IN DENMARK AQUIRED CASE,
that
was diagnosed with ringforms by simple microscopy of thin bloodsmears
back in 2001, which prompted me to researh this issue as much as I can!
I send the results to the local microbiology dep. as well as to SSI and asked for
proper evaluation, the response from the local microbiology dept. "it is too
controversial".
SSI offered inoculation, culture and PCR for
BORRELIA only (not mentioning anything about how to diagnose the
co-infections)
IN CASE I RELAPSED AFTER
TREATMENT, which was not yet begun, because the ID professor demanded certain
proof for all infections via a danish microbiologist, before he would
offer me any treatment for the three infections!
... when I did relapse badly, with spirochetes
in
my blood found twice within a year 2008 - by two different investigators and
10 months apart - I asked again, both the local microbiology dept. and SSI for the
test they had promised me back in 2001; the head of the local microbiology
dept. answered in wrtiting: no knowledge, no expertise in our dept., no
ressources, NO INTEREST! ... SSI "we do not do the test you ask for any
more and have no plan to do these methods in the future"! -
so THERE is no help for danish chronically ill patients, getting direct
test for Borrelia is NOT POSSIBLE - therefore I HAVE TO DO MY BEST to
help sick people!
A
danish saying "Need teaches a naked woman to make clothes" ... because
I did not get proper diagnostic help myself by my danish colleagues, was denied
tests described in the published literature, I was forced to
"do-it-myself" and to find another way, with help from good colleagues
abroad, and the experience gained from this, led me to do a little
research project on patients with similar history and symptoms.
In York UK 2004 Lyme conference I personally reported on 15 danish case
of ringforms detected in
thin blood smears
from until then 33 enrolled projects participants, who had Borrelia
antigen in their blood also, detected by direct immune stain for
Borrelia burgdorferi sensu lato: http://lymerick.net/York2004/ringform-DK.pdf
... (I am #1)
Overall
about 1/3 of a total of 50 project patients with "chronic Lyme"
symptomatology investigated, also had ringforms in their RBC,
and 75% of the 33 first "pilot project" participants had microscopic sign of one or more
co-infections PLUS borrelia antigen in their blood detected by direct
immunofluorescent stain specific for Borrelia burgdorferi sensu lato
... it was just a small pilot study with few patients (but what I could
do with very low economic and practical ressources, being sick myself on top), and it
was
not a treatment study, but as some patients recorded all the treatment
and symptoms (and many also came for repeated blood microscopiesby
me) during the course of treatment via Excel diary with
automatic
curve drawing, the improvement
on treatment could be calculated in these (too few) cases;
those who got relevant antibiotic treatment for BOTH found
co-infections AND borrelia IMPROVED OVERALL 50-75%,
usually within 3 months, some had further improvement
later; patients sick less than two years had a good chance
of
full recovery, but many had been sick for a very long time, overview over pilot project participants from the UK 2003 lecture: http://lymerick.net/York2003/projpatients.pdf
This
prompts for further investigation, which will cost a lot of money we
dont have, to do a proper controlled scientific investigation, but
nevertheless the case result that got diagnosed and responded well to treatment, were very, very promising!
NOTE: All
treatment studies for effect of antibiotic treatment have been done
only on
patients with negative direct test for Borrelia ... so of course there is little if any benefit from antibiotic treatment in these trials!
If we
really
want to evaluate results of antibiotic treatment for CURRENTLY ACTIVE Borrelia
infection, we must be 100% certain that the patient really HAS A
CURRENTLY
ACTIVE BORRELIA INFECTION!
... we
must be able to detect the Borrelia (antigen) infection reliably with
direct optimized methods (diary, microscopy, culture, PCR)
before
enrolling patients into a treatment study, because
enrolling
lots of cases with "post-Lyme"
persistent symptoms (nerve damage) with negative antigen test will of
course skew the results towards NO CERTAIN EFFECT in
proportion
with the group that does no longer have ACTIVITY CYCLE (weekly,
monthly)- previous studies like Klempners is pseudoscience, is not
useful to anybody, rather is very harmful to those really infected,
that are denied treament that can help them,
really!
Since
the project (stopped after 50 enrolled patients in 2007, for more
reasons) I found a few more cases with ringforms in RBCs, including the
latest, ON43, described above ...
UPDATED INFORMATION IS
NECESSARY TO INCREASE OUR DOCTORS AWARENESS - therefore I write
this little "review" ...
Probably
tick bitten (long term) sick patient, that do not regain their health
quickly after conventional treatment for Borrelia
infection, should NO LONGER be dismissed as malingerers or
psychosomatic cases, but should be investigated thoroughly and
be
properly investigated as "best science" show us is possible for both Borrelia
culture in BSK-H
(PCR subtyping possible after, rarely possible on fresh patient sample
due to very low amount of spirochaetes catched in the sample), Line
Western blot serology analysis (can detect many different antibodies,
including strain specific) plus be properly evaluated for the
many
other tickborne co-infections, that we now know that
ticks
may harbour and can infect humans and pets and farm
animals with (how about eating relatively raw meat as has
become
modern?), using the new types of PCR tests described in the
articles below, that are able to detect all subtypes of tickborne
patogens, and using locally found variant strains in IFA ...
until
we know more about what we have, and can develop more specific serology
test methods!
Any laboratory worker (any doctor really) should learn
and be able to do buffy-coat smears and stain blood
with
Giemsa and examine in the microscope, therefore I explain how and why
in this article!
Babesia venatorum (EU1)
PubMed search for Babesi*+(EU1+OR+venatorum): http://www.ncbi.nlm.nih.gov/pubmed?term=Babesi*+(EU1+OR+venatorum)
Molecular Characterization of a Non–Babesia divergens
Organism Causing Zoonotic Babesiosis in Europe. (2003)
http://wwwnc.cdc.gov/eid/article/9/8/02-0748_article.htm
"Subsequent testing of serum specimens from both [Babesia EU1] patients
showed IFA reactivity to B. divergens but not to B.
microti antigens; serum from the Italian patient was also
tested for reactivity to WA1 antigens and was negative."Patient characteristics table:
(CDC
unfortunately had no luck in producing infection in jirds by the
experimental inoculation of patients blood, so the IFA was done with B.
divergens as test antigen!)
The italian patient: "Titers of 1:64 (specimen from October 28, 1998) and 1:256 (February 15,
1999) in testing at both CDC and the Clinical Institute of Hygiene of
the University of Vienna"
The Austrian patient: "Titers of 1:256 (July 31, 2000) and 1:1,024 (August 8, 2000) in testing
at CDC and titers of 1:64 (July 31) and 1:1,000 (August 8) in testing at
the Clinical Institute of Hygiene of the University of Vienna" For comparison: The danish patients titer to unknown test antigen was 1:125 ...
[Babesiosis in an immunocompromised German patient]. (tysk, 2008)
http://www.ncbi.nlm.nih.gov/pubmed/18270666
"Babesiosis was confirmed by polymerase chain reaction (PCR) and the
parasite was identified as EU1. Serology
was negative. Therapy with clindamycin and quinine induced
remission. Following a relapse, retreatment with atovaquone and
azithromycin was initiated. After several months, seroconversion
occurred and the patient cleared the parasite 8 months after first
admission."
Transport of Babesia venatorum-infected Ixodes ricinus to
Norway by northward migrating passerine birds. (2011):
http://www.ncbi.nlm.nih.gov/pubmed/21699719
http://www.actavetscand.com/content/pdf/1751-0147-53-41.pdf
(I had problem with the version saved in PMC!)
METHODS: Passerine
birds were examined for ticks at four bird observatories along the
southern Norwegian coast during the spring migrations of 2003, 2004 and
2005. The presence of Babesia
was detected in the nymphs of Ixodes ricinus by real-time PCR. Positive
samples were confirmed using PCR, cloning and phylogenetic analyses.
RESULTS: Of 512 ticks examined, real-time
PCR revealed five to be positive (1.0%). Of these, four generated
products that indicated the presence of Babesia spp.; each of these
were confirmed to be from Babesia venatorum (EU1). Two
of the four B. venatorum-positive
ticks were caught from birds having an eastern migratory route
(P< 0.001).
[hence the other two may have come from a southern route, perhaps they
flew over Denmark?]
CONCLUSIONS:
Birds transport millions of ticks across
the North Sea, the Skagerrak and the Kattegat every year.
Thus, even with the low prevalence of Babesia-infected ticks, a substantial
number of infected ticks will be transported into Norway each year. Therefore, there is a continuous risk
for introduction of new Babesia spp. into areas
where I. ricinus can survive.
Therefore, let
us look at the incidence of Babesia spp. detected in ticks, humans or
animals in other European countries, located south of Denmark, from
where the passerine birds may have come and flying over Denmark on their
way to Norway, fouraging, resting underways ... leaving ticks or perhaps aquiring new ticks underways?
Ticks and associated
pathogens collected from domestic
animals in the
Netherlands.
(2007) (=6 mentioned above)
"Following an
outbreak of autochthonous canine babesiosis in the Netherlands, a
request made to veterinarians and the public to collect ticks from
companion animals resulted in 4298
ticks submitted between July 2005 and October 2006 to our
center.
Ticks were identified as
Ixodes ricinus adults (2907/4298, 67.6%), Ixodes sp. nymphs (529/4298,
12.3%) and Ixodes sp. larvae (385/4298, 9.0%), I. hexagonus adults
(328/4298, 7.6%), Dermacentor reticulatus (72/4298, 1.7%), and several
other exotic tick species such as Amblyomma flavomaculatum (formerly
Aponomma flavomaculatum), Hyalomma marginatum rufipes, Rhipicephalus
sanguineus, and R. turanicus (55/4298, 1.3%). Eight localities were
surveyed for the presence of local D.
reticulatus, a tick not indigenous to the Netherlands,
based on multiple submissions of D. reticulatus ticks from these areas.
D. reticulatus was collected from the vegetation in six of these
localities, confirming the presence of populations of this tick in the
Netherlands.
Adult I. ricinus (n=251),
I. hexagonus (n=237), and D. reticulatus (n=344) ticks were selected at
random and subsequently screened
by polymerase chain reaction (PCR) and reverse line blot (RLB)
hybridization for the presence of Borrelia, Babesia,
Theileria, Anaplasma, Ehrlichia, and Rickettsia species.
I. ricinus ticks were infected with Rickettsia
helvetica (24.7%), spirochetes
belonging to the Borrelia burgdorferi sensu lato group (7.2%),
the Ehrlichia-like "Schotii" variant
(2.4%) [Anaplasmataceae PDF
2004, suggested nomenclature for "Schottii variant"
is ‘Candidatus Neoehrlichia mikurensis’ acc. to fig. 2], Anaplasma phagocytophilum (1.6%),
Babesia sp. (EU1) (1.2%), Babesia divergens (0.4%),
and Babesia microti (0.4%).
[EU1:divergens/microti 3,5:1]
A. phagocytophilum (5.9%) and R. helvetica (0.8%) were also detected in
adult I. hexagonus ticks. Spotted
fever group Rickettsiae, previously reported as Rickettsia sp.
DnS14/RpA4 (14.0%), and Borrelia burgdorferi sensu lato (0.3%) were
detected in the D.
reticulatus ticks, which appeared to be free from B. canis
infection. We
concluded that a much broader spectrum of ticks and tick-borne
pathogens is present in the Netherlands than previously thought,
including several potential zoonotic pathogens."
The First Detection of Babesia EU1 and Babesia canis canis in Ixodes ricinus Ticks (Acari, Ixodidae) Collected in Urban and Rural Areas in Northern Poland (2009)
http://www.pjm.microbiology.pl/archive/vol5832009231
"Ixodes ricinus, the most commonly observed tick species in Poland, is a known vector of such pathogenic microorganisms as TBE viruses, Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum, Rickettsia helvetica, Babesia divergens and B. microti
in our country.
Our study aimed to find out whether this tick can also
transmit other babesiae of medical and veterinary importance.
DNA
extracts of 1392 ticks (314 nymphs, 552 male and 526 female ticks)
collected in urban and rural areas in the Pomerania province (northern
Poland), were examined by nested PCR for the detection of Babesia
spp., using outer primers: 5-22F and 1661R, and inner primers: 455-479F
and 793-772R, targeting specific fragment of 18S rRNA gene. Overall, at
least 1.6% ticks were found to be infected with babesial parasites. In
the case of nymphs, the minimal prevalence was 0.6%, and it was approx.
3-times lower than in adults (1.9%). Percentages of infected males and
females were comparable (2.0% vs. 1.7%). Sequences of 15/22 PCR-derived
fragments of 18S rRNA gene demonstrated 100% similarities with the
sequence of Babesia EU1 (proposed name B. venatorum) (acc. no. AY046575) (n=13) and with B. canis canis (acc. no. AY321119) (n = 2), deposited in the GenBank database. The partial 18S rDNA sequences of Babesia EU1 and B. c. canis obtained by us from I. ricinus
have been deposited in GenBank, accession nos. GQ325619 and GQ325620,
respectively. The results obtained suggest the possible role of I. ricinus
as a source of microorganisms, which have been identified as agents of
human and canine babesiosis, respectively, in Europe. To our knowledge
this is the first report on the occurrence of Babesia EU1 and B. c. canis in I. ricinus in Poland."
Babesia sp. EU1 infection in a forest reindeer,
The Netherlands.
(2011)
"All blood
and tissue samples from each organ tested were positive only for Babesia
sp. EU1. ...
Of I. ricinus ticks from the Netherlands, ≈1% are infected with Babesia sp. EU1 (6).
The only confirmed reservoir host of Babesia sp.
EU1 is roe deer (Capreolus capreolus) (9).
The infected forest reindeer resided in a zoo in an area without direct
contact with roe deer, although roe deer are abundant in the forests
surrounding the zoo."
Co-infection with
Borrelia species and other tick-borne pathogens in humans: two
cases from Poland. (2010)
"The
co-infection rate of these pathogens was found to be rather low
(Borrelia spp./Anaplasma
phagocytophilum – 4.2%, 1/24; Borrelia spp./Babesia spp. – 4.2%, 1/24).
However, due to the increased prevalence of Borrelia spp. in Ixodes
ricinus ticks in Poland and the recent emergence of new tick-borne
infections, it is
necessary to
carefully evaluate the true risk of human infection with several
pathogens using more sensitive and reliable diagnostic tools.
This
is the first report of human infection with Babesia spp. in Poland that
has been confirmed by molecular techniques with homology of 98.9% to B.
divergens or Babesia EU1."
Excerpts:
"Three
recently described human cases identified in Italy, Austria and Germany
were caused by infection with a species named EU1, exhibiting molecular
characteristics distinct
from those
of B. divergens [16, 18]. The
rodent species B. microti is also present throughout Europe, although
there has only been one verified case of human babesiosis due to
infection with this species [20]. In recent years, co-infections of
humans acquired from Ixodes ricinus ticks have been observed quite
frequently in the United States [1, 4, 38] and in Europe [24, 44].
However, only three such cases have been reported so far in Poland [12,
17, 27].
In this retrospective study we examined Borrelia-seropositive
individuals from southeastern Poland, where I. ricinus ticks are highly
endemic, for coinfection with A. phagocytophilum and Babesia species."
"Examination
of a smear of the Babesia-positive blood sample stained with Giemsa
revealed Babesia spp. infection with a very low level
parasitemia of 0.02% (Fig. 3)." ...
"Human
co-infections with the pathogens Borrelia spp. and A. phagocytophilum
with clinically (erythema migrans) and serologically (seroconversion)
confirmed Lyme borreliosis as well as asymptomatic anaplasmosis
(positive serology or PCR for A. phagocytophilum) have been described
in Poland [12, 17] and other countries [23, 25]. Nevertheless, the few previous reports
of such co-infections indicate that the resulting disease is more
severe and prolonged [39*].
Since the late 1950s, two species of Babesia, B. divergens from cattle
in Europe and B. microti from rodents in the northeastern and upper
midwestern parts of the USA, have been shown to infect humans [26]. The
species B. microti, B. divergens, B. odocoilei-like, and Babesia EU1
are known to be prevalent in I. ricinus ticks across Europe, including
Poland [9, 15, 33, 43]." [no clinical case description]
[39*] = Coinfections acquired from ixodes
ticks. 2006 PDF ..
NB
In this review I found more wrong statements that has been disproven,
so read carefully and check all statements, that may
now be out of date!
For
instance the authors claim that ONLY Bbss, B. afzelii and B.
garinii are known to be human pathogenic species.
However, cases of
other Borrelia burdorferi sensu lato had already been detected and
reported before publication of the review:
Borrelia bisettii (Strle F, Slovenia 1999 PM "Thus far four Borrelia
species were found by isolation to cause disease in humans: B. afzelii,
B. garinii, B. burgdorferi sensu stricto, and B. bissettii.")
B. lusitaniae,
Portugal 2004 PDF Borrelia valaisiana
Greece, 2004 HTM Borrelia spielmanii
(so far only detected in early Lyme disease AFAIK?, i.e. in EM rash)
2005 PDF
-
so the authors were clearly not fully up-to-date with the published
literature (or were ignorant?) when they wrote that statement in
2006!
Proving
causation is not possible after 10 years of chronic
illness, as in the cases with B. lusitaniae and B. valaisiana, however
both these patients were described as clinically
compatible with
what some of us
(ILADS stance) call "chronic Lyme disease", while other
colleagues
(of IDSA stance)
stubbornly deny the existence of persistent borrelia infection
in
"chronic Lyme disease", where they usually mean "typical
NEURO-borreliosis" (ass. w/ B. garinii infection), despite there are
lots of
culture, PCR and/or microscopy verified LATE and POST-TREATMENT cases
that has been described since 1989; because the IDSA
chronic
Lyme denialists
usually do not reference any of the published works, that speak against
their stance (ignorance seem to fluorish among them), I made a
list of references - persistent-borreliosis -
and put it on my website in 2003, to make it easier for
myself and others to check up on which references are
MISSING
/ not discussed in articles authored by folks of IDSA stance ;)
A more
recent review over Lyme borrelia diversity is discussed by Stanek
(EUCALB, usually stick close to the IDSA stance in US) in "The
expanding Lyme
Borrelia complex—clinical significance of genomic species?" (1911) PDF .. he
still
claim that only the 3 old wellknown Borrelia genospecies known
from 1992 are with
certainty humane pathogenic, about the others he
describe them as "single" or "a few case reports", like on page
491, he writes "the presence of B. valaisiana [in neuroborreliosis] was
confirmed in three cases [1]."
So I
wonder, HOW many cases does it take for them to accept that
Borrelia infection sometimes can be a chronic persistent
perhaps
life-long infection, especially in some susceptible
individuals?
The recently published NIH-primate
study by Embers et al. (Full text + PDF) demonstrate
long term persistence of Borrelia despite 3 months ceftriaxone
treatment (error in drug was corrected), this has not yet been added to
my persistent borreliosis
reference list!
"Rhesus
macaques were infected with B. burgdorferi and a portion received aggressive antibiotic
therapy 4–6 months later.* Multiple methods
were utilized for detection of residual organisms, including the
feeding of lab-reared ticks on monkeys (xenodiagnosis), culture,
immunofluorescence and PCR. Antibody
responses to the B. burgdorferi-specific C6 diagnostic peptide were
measured longitudinally and declined in all treated animals.
B. burgdorferi antigen,
DNA and RNA were detected in the tissues of treated animals.
Finally, small numbers
of intact spirochetes were recovered by xenodiagnosis from treated
monkeys. These
results demonstrate that B. burgdorferi can withstand antibiotic
treatment, administered postdissemination, in a primate host."
*
many
humans have been sick for much longer, over a year to
several years, before getting diagnosed
and treated first time for their Borrelia infection;
esp. those with B.
afzelii here in Europa are diagnosed late, because focus is
set
only on detecting "typical" neuroborreliosis [Bannwarth syndrome] with
high spinal-Borrelia-antibody index etc. - which is characteristic for
B. garinii neuroborreliosis, but not in B. afzelii neuroborreliosis
(Strle CID 2006 PDF)
As long as genotyping it not yet
generally avaiable to us for diagnosis we will never know with
certainty what type of Borrelia(s) the patient got infected with!
We
need access to culture for Borrelia (at least in cases that do not get
well on standard tretment for Borrelia) as well as co-infections in
cases suspect of chronic late or persistent Lyme borreliose, where
antigen specific diagnosis of Borrelia will support necessity for
treatment and aid in choice of treatment.
MY VIEWPOINT IS:
Up until
now so far 18 named genospecies have been placed in
the Borrelia burgdorferi sensu lato (Bbsl) spp. complex, and
so
far 8 of these have been detected in material from human Borreliosis
cases from Europe since 2004! - the enlarging spectrum show us
that other strains of borrelia than the 3 old one, may eventually turn
out be humane pathogenic, could perhaps not be detected before
the
PCR era, because these strains are so antigenically different,
that the commonly used commercial Borrelia antibody tests, that are
based on antigens from the 3 old types of Borrelia burgdorferi sensu
lato, may not detect antibodies directed against the new types; more of
the cases with other types have shown to been seronegative or
very
low seropositive, despite they harbour Borrelia!
Previously
detectable Lyme antibodies have been shown to disappear, despite
culture verified persistence of Borrelia in ligamentous tissue (Häupl
et al. and more) ...
During very active Lyme borreliosis with
detectable spirochetes by simple microscopy, many patients may test
seronegative in same plasma fraction, which can be explained by immunecomplex bound antibodies
are not being measured: http://kroun.ulmarweb.dk/Borrelia-IC.html
(in danish, but reference mentioned are in english) ... some of them will
"seroconvert" 1-2 month into clinically effective antibiotic treatment,
when there is no longer antigens in blood as often (shift to monthly
relapse cycle); when treatment is stopped some of them relapses again,
and again spirochetes can be detected, and they may turn seronegative
.. retreatment usually works and they may "seroconvert" again, when the
cycle shift to monthly .. UNDERSTANDING THE DYNAMICS OF TEST
RESULTS IN RELAPSING INFECTION IS A NECESSITY!
Because
IDSA base the very diagnosis of Lyme borreliosis primarily on that the
patient MUST HAVE POSITIVE SEROLOGY, two-tier, because they have chosen
to use the CDC EPIDEMIC SURVEILLANCE CRITERIA as DIAGNOSTIC CRITERIA
for Lyme borreliosis in the USA, despite CDC actually have warned
against it! - this BLOCK for openminded discussion on the many cases of
proven persistent and othen seronegative borreliosis cases. They
have
chosen to define"Lyme disease" in a certain way, not accepting a
broader perspective; most of the
controversies arises from the fact that some of the lead
authors
in IDSA on Borrelia have personal conflicting economic interest in
serology tests!
From my viewpoint I talk about "Borrelia
infection" in a much broader sence, i.e. the patient is infected, but
not necessarily clinically ill, when ANY borrelia spp., broad defined
i.e. including TBRF and LBRF sub-spp., can be detected by
direct
test methods like culture, PCR and/or microscopy (plus the patient
has history indicative of borrelia like known tickbites, EM
rashes, previously positive serology test) ...
Borrelia infection
(antigen present and able to revert and cause symptoms, but is
currently an inactive infection) may be latent, asymptomatic for weeks,
months to even many years, just like in tertiary syfilis, there may be
a potential risk of later relapse, in case of immune depression; such
have been described in persistent borreliosis list, there are lots of
published reports that supports this, really!
The KEY POINT in
diagnosis of ACTIVE INFECTION is EASY, SIMPLE namely the RE-OCCURRENCE
OF THE CYCLICAL CLINICAL RELAPSE PATTERN, WHICH ALLOWS US TO
DETECT SPIROCHETES IN BLOOD BY MICROSCOPY DURING THE FIRST DAY
OF
EVERY ACTIVE FLARE PERIOD! .. in blood taken at other times spirochetes
are usually not detectable!
If the patient has a "weekly" relapse
pattern spirochetes can be detected in 1 per 10 days of the
cycle
(10% chance of lucky catch, while the risk of missing the catch is
90%); in patients on a monthly cycle 1 per 30 days, the chance
of
lucky catch is 3%, while the chance of missing the catch is
97%)
if the clinician does NOT time the sampling for direct
detection
to happen day one of a new flare!
The antigen in my project
cases reacted with added Borrelia antibody that had been
absorbed
for TBRF species and tested by the producer to be specific for Borrelia
burgdorferi sensu lato (KPL).
First when we know which strain
the patients have got and can compare to clinical status, it will
become possible to evaluate the clinical significance of the different
strains of Lyme borreliae!
Let us spare the discussion now and agree
that we do not yet have information enough to make a definite
conclusion on this issue, that would serve the sick best to keep an open mind to what may be possible, I think!
- there may be strains out there that has not even been found yet ...
The
authors [of 39] also claim that larvae of Ixodes is not transovarially
infected with Borrelia. However I know more studies proving
that
if the mother tick is systemically infected with Borrelia (which from
5-30% have been found to be) the Borrelia infection will be transmitted
to 100% of eggs and the progeny larvae will be born infected (for
instance old works by Burgdorfer himself, I have not made a list yet,
is on my to-do-list)! ... More PCR studies on unfed Ixodes
larvae
have shown that 5-10% may harbour Borrelia spp. and therefore
is
potentially contagious!
There may be more disproven out-of-date
or skewed statements in articles depending on "stance" of the
authors, so
read articles with great caution! .. be very thorough and
check
reference-list, not only if they reference correctly, but also which
articles are missing, that they should have referenced and discussed in
their context, really!
Occurrence of Babesia spp., Rickettsia spp. and
Bartonella spp. in Ixodes
ricinus in Bavarian
public parks, Germany.
http://www.ncbi.nlm.nih.gov/pubmed/21762494
http://www.parasitesandvectors.com/content/pdf/1756-3305-4-135.pdf
"The following prevalences were detected:
Babesia spp.: 0.4% (n =
17, including one pool of two larvae) in 2009 and 0.5 to 0.7% (n = 11,
including one pool of five larvae) in 2010;
Rickettsia
spp.: 6.4 to 7.7% (n = 285, including 16 pools of 76 larvae) in 2009.
DNA of Bartonella
spp. in I. ricinus in Bavarian public parks could not be identified.
Sequence analysis revealed the following species: Babesia sp. EU1
(n = 25), B. divergens (n = 1), B. divergens/capreoli (n =
1), B. gibsoni-like (n = 1), R. helvetica (n = 272), R. monacensis
IrR/Munich (n = 12) and unspecified R. monacensis (n = 1).
The majority of coinfections were R. helvetica with A. phagocytophilum
(n = 27), but coinfections between Babesia spp. and A. phagocytophilum,
or Babesia
spp. and R. helvetica were also detected."
=>
Ratio of Babesia venatorum (EU1):Babesia divergens found in
the Bavarian park ticks was 25:1
i.e.
humans and pets visiting that city park is at 25 times
relative
higher risk of getting infection with Babesia EU1 compared to Babesia
divergens, that is, if they get a Babesia infection, since
less
than 1% of the examined ticks were found infected with Babesia species
the general risk of aquiring Babesia infection is
still quite low!
Presence of potentially
pathogenic Babesia sp. for human in Ixodes
ricinus in Switzerland.
(2006)
http://www.ncbi.nlm.nih.gov/pubmed/16841874
http://www.aaem.pl/pdf/13065.pdf
"The
serological assays presently available are not able to discriminate
between EU1 and B. divergens infections [14], hence all the cases
reported to date have been ascribed to B. divergens."
"We
have described a new PCR method based on the 18S rRNA gene that allows
the detection of Babesia in I. ricinus ticks and in blood samples. The amplified fragments
show an important number of mutations.
In addition, the variability of the target fragments (25.3%) is similar
to the variability of the complete gene sequences (20.3%). Because of this variability, the
short amplicon of 411-452 bp of the 18S rRNA gene may be used for
accurate species resolution.
Indeed, we could differentiate all the Babesia species we considered
(Fig. 2). Moreover, in the B. canis cluster, we clearly could separate
the two subspecies B. canis vogeli and B. canis canis (Fig. 2). The
analytical sensitivity of the PCR assays has been shown to be 27.5
fg/reaction of B. divergens DNA. However, the majority of the preview
reports describing PCR for the detection of Babesia in ticks did not
report the sensitivity, with the exception of a study which showed a
limit of detection of 50 organisms/ml of canine
whole blood [3]. ...
This is the first study
reporting the presence of Babesia sp. EU1 in Switzerland.
This organism was detected in ticks collected in two areas located
North (Neuchātel) and South of the Alps (Ticino). The presence in
Switzerland of B. microti and B. divergens was also confirmed. ...
Recently,
Meer-Scherrer et al. [20] described the firsthuman case due to B.
microti in a Swiss patient. However, the behaviour and the virulence of
the European B. microti strains might be different from those of North
America. Interestingly, in another study, it has been suggested that B.
microti in the United States may be more virulent for humans than the
B. microti-like isolated in Japan [25]. It is important to consider
that B. microti actually consists of a genetical species complex based
on the 18S rRNA gene: Babesia isolated in rodents divide in two clades,
one zoonotic, including our samples, and the other maintained only in
rodents [11].
Recently, two cases of human babesiosis, in Italy and
Austria, have been attributed to EU1 [14]. Moreover, in Slovenia ticks
have been found infected by this species [6]. The EU1 sequences
obtained in these different countries, including Switzerland, are
identical or very close (99.8% of similarity) and thus no specific
geographic association may be identified. An important issue concerning
Babesia sp. EU1 is to determine whether it is an emergent species."
First molecular evidence
of potentially zoonotic Babesia microti and Babesia sp. EU1 in Ixodes
ricinus ticks in Belgium.
(2011)
"We report the first molecular
evidence of the presence of Babesia sp. EU1
and Babesia
microti in Ixodes ricinus ticks in Belgium. A 1-year national survey
collected 1005 ticks
from cats and dogs. A polymerase chain reaction technique
amplifying a part of the 18S rRNA gene detected Babesia spp. in 11 out of
841 selected and validated tick extracts.
Subsequent sequencing
identified Ba.
microti (n=3) and Babesia sp. EU1 (n=6). This study
has demonstrated a low infection rate (1.31% with 95% CI: 0.65-2.33) of
Babesia
spp. carriage in I. ricinus ticks in Belgium but, for the first time,
reports two potentially zoonotic species belonging to this genus. Coinfection with Ba. microti and
Borrelia burgdorferi sensu stricto also was demonstrated.
In addition, this study clearly demonstrates that inhibitors of polymerase chain
reaction amplification are present in engorged ticks."
Questing ticks in
suburban forest are infected by at least six tick-borne pathogens.
[France,
2011]:
"...
Pathogen prevalence rates were evaluated by polymerase chain
reaction detection and sequencing in questing ticks, individually for
adults and in pools of 10 for nymphs. In addition to finding
micro-organisms corresponding to symbionts, we found high prevalence rates of B. burgdorferi s.l.
(32% of adult females and 10% of nymphs)low to moderate ones of Anaplasma phagocytophilum
(~1%), spotted
fever group Rickettsia
spp. (~6%), Babesia sp. EU1 (~1%), Bartonella birtlesii (0.1%), and
Francisella tularensis (!1%).
Our findings extend the knowledge of the geographical distribution of
these endemic and emergent pathogens and support the conclusion that ticks
are important vectors of pathogenic micro-organisms in suburban
forests. Moreover, tick
coinfection with multiple pathogens was found to occur frequently,
which poses a serious challenge for diagnosis and appropriate treatment.
The incrimination of these pathogens in potentially severe pathologies
requires widespread surveillance to assess the risk of infection,
thereby facilitating diagnosis and treatment, as well as raising local
awareness of tick-borne diseases." and
Detection and
characterization of Babesia species in Ixodes
ticks in Estonia. (2011)
"The presence
of Babesia
spp. was studied in 2603 Ixodes ricinus and Ixodes persulcatus ticks
collected at seven sites in Estonia. By reverse line blot screening,
spp. was detected in 36 (1.4%) ticks, among them 18 (0.7%) were further
recognized by a BabesiaBabesia microti probe, 3 (0.1%) by a Babesia divergens probe, and the other
15 (0.6%) were recognized only by the universal Babesia spp. "catch all" probe.
Sequence
analyses of 6 of these 15 samples revealed that all of them belonged to
Babesia sp. EU1. B.
microti was detected in both tick species I. ricinus and I. persulcatus
at the seven sites, whereas B. divergens-like and Babesia sp. EU1Babesia sp. EU1 share a
high rate of similarity and are closely related to sequences from other
European countries, Siberia, and United States. The present study
demonstrated for the first time the existence and distribution of Babesia spp.
in I. persulcatus and I. ricinus ticks in Estonia."
Prevalence of three
zoonotic Babesia species in Ixodes
ricinus (Linné, 1758) nymphs in a suburban forest in Switzerland.
(2011)
"... The aim
here was to determine how frequently these [Babesia] species infect I.
ricinus nymphs in a suburban forest and to determine their prevalence
over 3 years along a pathway delimited in four different sections.
Babesia spp. was detected and identified
in 44/2568 (1.7%) I. ricinus nymphs using Reverse Line
Blot.
B. venatorum
[EU1] was infecting 1.1% (27/2568) of nymphs, B. divergens
0.2% (4/2568), and B. microti 0.7% (13/1908).
Tick infection rates by these three Babesia species between years were not
different except for B. microti, which was significantly less frequent
in ticks in 2008 than in 2006 and 2007 according to a test using
trusted intervals of percentages. B. microti was displaying the greater
difference of prevalence among sampling sections, ranging from 1.6% in
section 1 to 0% in section 4. The
presence of these three Babesia species that are of medical
relevance in a suburban forest where I. ricinus tick density is high
requires attention from physicians, particularly for patients
presenting unspecific symptoms and for patients who are
immunocompromised, and who have history of contact with tick biotopes."
Pathogens of emerging
tick-borne diseases, Anaplasma phagocytophilum, Rickettsia spp., and Babesia spp., in Ixodes
ticks collected from rodents at four sites in Switzerland
(Canton of Bern). (2011)
"The
prevalence of Babesia
spp. reached 2.4% and identification at the species level revealed B. venatorum (1.7%) and B. microti (0.4%)."
Occurrence and
identification of risk areas of Ixodes
ricinus-borne pathogens: a cost-effectiveness analysis in north-eastern
Italy.
(2012)
"Eleven pathogens were detected
in 77 out of 193 ticks collected in 14 sites. The most
common microorganisms detected were Borrelia
burgdorferi sensu lato (17.6%), Rickettsia helvetica (13.1%), and "Ca.
N. [N= Neoehrlichia] mikurensis" (10.5%).
Within the B. burgdorferi complex, four genotypes (i.e., B. valaisiana,
B. garinii, B. afzelii, and B. burgdorferi sensu stricto) were found.
Less prevalent pathogens included R. monacensis (3.7%), TBE virus
(2.1%), A. phagocytophilum (1.5%), Bartonella
spp. (1%), and Babesia EU1 (0.5%).
Co-infections by more
than one pathogen were diagnosed in 22% of infected ticks."
Antibody prevalence and
molecular identification of babesia spp. In roe deer in
France.
(2012)
"In a
region-wide serologic study carried out in 2004 on free-ranging hunted
roe deer in various landscapes, we found that 58% of the animals (237 out of
406) were antibody positive for Babesia divergens antigen.
Serologic and infection status was also analyzed for 327 roe deer
live-trapped in two fenced forest areas over 5 yr (2004-08). For two consecutive years during
this period, 92 and 94% of the deer in these closed populations were
antibody-positive for B. divergens.
Babesia spp. were isolated in autologous
red blood cell culture for 131 of the trapped animals (40%).
Molecular typing was done on 76 isolates with polymerase chain reaction
(PCR)-restriction fragment length polymorphism methods targeted at the
18S ribosomal subunit gene (18 isolates) and the Bd37 gene coding for a
merozoļte surface antigen implicated in a protective response (60
isolates).
Results indicated
continuous cocirculation of B. capreoli and B. venatorum in both forests and possible
coinfection of animals with both species. No infection with B. divergens
was detected. Fifteen isolates were confirmed to be B.
capreoli by sequencing part of the 18S rRNA gene. Using PCR detection
of the Bd37 gene, all nine isolates of B. venatorum in this study were negative,
whereas the 15 confirmed and 50 putative B. capreoli isolates showed
very variable restriction profiles, distinct from those known for Bd37
in B. divergens. Two
isolates showed conflicting results, suggestive of mixed infection."
Babesia
duncani (WA1)
PubMed Babesi*+(WA1+OR+duncan*):
http://www.ncbi.nlm.nih.gov/pubmed?term=Babesi*+(WA1+OR+duncan*)
This
strain is described as Babesia-gibsoni-like and is known to cross react
in serology test with Babesia gibsoni, while patients with
Babesia
WA1 usually test negative on antibody test for B. microti!
- above ref. from the German city park, described finding 1 Babesia gibsoni-like!
... perhaps it is closely related to be called WA1-like, since
WA1 is also B. gibsoni-like?
I need mention this because sign of WA1 infection has been
described in Europe ONCE, and in Denmark of all places:
1996 danish conf. abstract: 14 of 132 (10,6%) of danish
neuroborreliosis patients and 2 of 50 donors tested seropositive for
WA1 antibodies:
"Two of the patients with elevated titers to
WA1 had a prolonged disease course, one with elevated liver enzymes."
The third described case
of transfusion-transmitted Babesia duncani. (2011).
http://www.ncbi.nlm.nih.gov/pubmed/22168221
Molecular
and indirect fluorescent antibody (IFA) analyses were negative for B. microti
but were positive for B. duncani (IFA titer, 1:1024).
The complete 18S ribosomal RNA gene of the parasite was amplified from
a blood specimen; the DNA sequence was identical to the sequence for
the index WA1 parasite isolated in 1991.
The patient's case prompted a transfusion investigation: 34 of 38
pertinent blood donors were evaluated, none of whom tested positive by
B. microti IFA.
The implicated donor - a 67-year-old California resident - had a B. duncani titer of 1:4096; B. duncani also was isolated by inoculating
jirds (Mongolian gerbils) with a blood specimen from March 2009, more
than 10 months after his index donation in April 2008. The patient's
case was diagnosed more than 4 months after the implicated transfusion
in May 2008.
THIS IS SO INSTRUCTIVE that I do not find it necessary to list other
reports that likewise show that
commonly used assays for B. microti does not detect infection with B.
duncani (WA1)
It
shows the timing, long persistence of babesia if not treated, risk for
blood recipient if the asymptomatic low level carrier decides to donate
blood.
There are no screening for Babesia, blood banks just
ask if the patient feel healthy, do not exclude previous borrelia /
tickbitten as donors, do not apply any tests for Babesia on donors
blood.
The Japanese donor transmitted case -
http://lymerick.net/York2004/Japanese-donor-Babesia.htm
http://www.ncbi.nlm.nih.gov/pubmed?term=babesi*+Japan+donor
-
http://jcm.asm.org/content/38/12/4511.full.pdf
"The donor's serum exhibited
a high antibody titer against the isolate from the patient, whereas it
exhibited only a weak cross-reaction against B. microti strains
isolated in the
United States."
Indirect
fluorescent-antibody test (IFAT). An approximately 50%
suspension of infected RBCs which had 30 to 50% parasitemia was
prepared in phosphatebuffered
saline
(PBS; pH 7.2) containing 50% fetal bovine serum. Roughly
0.3-ml
aliquots were spread into each well of a 24-well HT Coating Slide (MS
342 BL; Bokusui Brown, Tokyo, Japan) and were then dried. The slides
were fixed in acetone for 5 min and were then immediately transferred
into PBS to lyse the RBCs. Following removal of solution by briefly
blotting with a filter paper, the slides were placed in a moisturized
chamber, and 15 ml of serial twofold dilutions of serum specimens,
starting from 1:25, was added to each well. After 1 h of incubation at
room temperature, the slides were washed in PBS, and 15 ml of
fluorescein isothiocyanate-labeled protein A (EY Laboratories, Inc.,
San Mateo, Calif.) diluted 1:200 in 5% fetal bovine serum–PBS was added
to each well. The slides were incubated at room temperature for 1 h and
washed in PBS. Component A of the Slowfade antifade kit (Molecular
Probes, Eugene, Oreg.) was mounted onto each well, and cover glasses
were placed on the slides. Fluorescent parasites in RBCs were observed
with a fluorescence microscope at a magnification of 3200.
Reference
strains of B. microti. The Gray strain (4) was a gift from
J. Dickerson, Division of Parasitic Diseases, Centers for Disease
Control and Prevention.
Strain
Gray-Mo, a mouse-adapted substrain of the Gray strain, has been
described previously (15). The GI and AJ strains were provided by H.
Saeki, Nippon Veterinary and Animal Science University. Syrian hamsters
were used for propagation of the Gray strain, and the antibodies in
their convalescentphase sera were used as the specific antibodies. The
Gray-Mo, GI and AJ strains were propagated in C.B-17 scid mice, and
antisera against these strains were prepared with BALB/c mice.
-
shows another problem, that there may be local variants in different
places of the world, where strains of genetically similar
strains, differ enough from each other in
surface antigens, to not be readily detected by a B. microti
anitbody test based on a strain from the USA!
=> test should be done with local microbe
strains!
Babesia divergens–like
Infection, Washington State (2004)
http://wwwnc.cdc.gov/eid/article/10/4/03-0377_article.htm
Most reported U.S. zoonotic cases of babesiosis have
occurred in the Northeast and been caused by Babesia microti.
In Washington State, three cases of babesiosis have been reported
previously, which were caused by WA1 (for “Washington 1”)-type
parasites.
We investigated a case of babesiosis in Washington in an 82–year-old
man whose spleen had
been removed and whose parasitemia level was 41.4%.
The complete 18S ribosomal RNA gene of the parasite was amplified from
specimens of his whole blood by polymerase chain reaction.
Phylogenetic analysis
showed the parasite is most closely related, but not identical, to B. divergens (similarity score, 99.5%), a
bovine parasite in Europe.
By indirect
fluorescent-antibody testing, his serum reacted to B. divergens but not to B. microti or WA1 antigens.
This
case demonstrates that babesiosis can be caused by novel parasites
detectable by manual examination of blood smears but not by serologic
or molecular testing for B.
microti or
WA1-type parasites.
THE KEY POINT! ...
Neg.
antibody test and negative PCR test can not be used to rule out Babesia
infections, particularly NOT when ringforms can be detected by simple
microscopy, which still remains "the gold standard"
test!
Negative thin and thick blood smears can not be used to rule o
Malaria:
Wikipedia om Malaria detection methods: http://en.wikipedia.org/wiki/Malaria_antigen_detection_tests
QBC Quantitative buffy-coat method:
PubMed QBC+Malaria:
http://www.ncbi.nlm.nih.gov/pubmed?term=QBC+Malaria
QBC Malaria:
http://www.malariasite.com/malaria/QBC.htm
Note:
The QBC is as screen method fast and more sensitive, but if
positive by QBC normal thin and thick blood smear examinations
should also be done, for optimal strain identification
List of references on the
use of QBC Malaria method:
http://www.qbcdiagnostics.com/products/fm/malaria/studies.asp
Application and
Evaluation of QBC Malaria Diagnosis in a Holoendemic
Area. (1994)
http://www.ncbi.nlm.nih.gov/pubmed/7812314
"QBC proved
more sensitive than the thick-film method, detecting - on day 14 of the
in vivo test - low parasitaemias that had gone undetected by thick
film. Lastly, this study reports on the conversion of QBC readings
(parasitaemia per field) into thick-field terms (number of parasites
per microliter of blood), with the aim of measuring the degree of
recurring parasitaemia."
Diagnosis of Malaria by
Acridine Orange Fluorescent Microscopy in an Endemic Area of Venezuela
(1996)
http://www.bioline.org.br/pdf?oc96013x
Detection of
Plasmodia in Acridine Orange Stained Capillary Tubes (The
QBC System).
(1990)
http://www.ncbi.nlm.nih.gov/pubmed/2098913
Malaria Diagnosis. A
brief review. (2009)
http://www.ncbi.nlm.nih.gov/pubmed/19488414
http://www.parasitol.or.kr/kjp/Synapse/Data/PDFData/0066KJP/kjp-47-93.pdf
"Nowadays, portable fluorescent microscopes
usinglight
emitting diode (LED) technology, and pre-prepared glass slides with
fluorescent reagent to label parasites, are available commercially
[38]. Although the QBC technique is simple, reliable, and
user-friendly, it requires specialized instrumentation, is more costly
than conventional light microscopy, and is poor at determining species
and numbers of parasites."
38: Partec reagents and accessories. http://www.partec.com
http://www.partec.com/reagents-accessories/essential-healthcare/reagent-kits.html
05-8951 Partec Rapid Malaria
Test, 200 tests
Ready-prepared and ready-to-use Malaria test slides with
dried-in reagents for easy-to-perform 1-step protocol and immediate
analysis. Maximum shelf life: 12 months.
Evaluation of the QBC
Method to Detect Malaria
Infections in Field Surveys. (1994)
http://www.ncbi.nlm.nih.gov/pubmed/7925060
"We conclude
that the QBC is quicker, with high sensitivity, and will prove useful
in clinical and epidemiological screening, especially when parasitaemia
is low."
Direct acridine orange
fluorescence examination of blood slides compared to current techniques
for malaria diagnosis. (1996)
http://www.tropicalmedandhygienejrnl.net/article/S0035-9203%2896%2990300-4/abstract
"The renewed
interest in the use of fluorochromes for malaria diagnosis prompted us
to evaluate the acridine
orange fluorescence technique on blood slides, and to
compare it with
established techniques using thick and thin blood films
and the QBC(TM) malaria
test, using the Giemsa-stained thick film technique as our
standard method for comparison.
We compared 123 positively diagnosed cases and 120 negative cases.
For primary samples (day 0), the sensitivity of the thin blood film
fluorescence acridine orange technique (AO) was 96·4%, and its
specificity was 95·1%.
In cases of imported malaria, with a prevalence rate of 16·2%, the
positive predictive value was 79·2% and the negative predictive value
99·3%.
Sensitivity of AO was
significantly higher than that of Giemsa-stained thin blood films for
parasitaemias <5000/μL. The potential of AO for species
diagnosis of Plasmodium was 85·2%, using Giemsa-stained
thin films as the reference technique.
Where QBC(TM)
imposes a cost limitation, especially in developing
countries, despite its high performance, the AO diagnostic technique is a
valuable alternative, because of its simplicity, almost negligible
cost, and its diagnostic reliability. The method may also have
potential value in the diagnosis of other microbiological diseases.
Feasibility and
limitations of acridine orange fluorescence technique using a Malaria Diagnosis Microscope in Myanmar.
http://www.ncbi.nlm.nih.gov/pubmed/12530504
http://www.lib.okayama-u.ac.jp/www/acta/pdf/56_5_219.pdf
About: "Acridine Orange Technique.
AOTF were stained by placing a small amount of AO solution in a single
strip down the center of the cover slip (18 x 18 or 24 x 24 mm) that
was laid on a filter paper or a paper towel. Holding the thick
blood-smeared slide at both ends with the smear facing downwards, the
technician pressed the slide gently against the cover slip with AO
stain. Any excess stain squeezed out was adsorbed by the underlying
filter paper or paper towel. The blood smear was examined immediately
under an MDM-ESL microscope starting from low magnification 200 X,
where parasites could be easily spotted. The parasites were best
identified under 600 X magnification."
(preliminary .. there may be more interesting articles .. check back later)