STUDIES ON THE LIFE CYCLE OF SPIROCHETES*
VIII.
SUMMARY AND
COMPARISON OF OBSERVATIONS ON VARIOUS ORGANISMS
EDWARD D. DELAMATER, M.D., Ph.D., MERLE HAANES, M.D.,
RICHTER H, WIGGALL, M.D., AND DONALD M. PILLSBURY, M.D.
THE JOURNAL OF INVESTIGATIVE DERMATOLOGY 1951; 16:
231-56
From the Department of Dermatology and Syphilology,
University of Pennsylvania
Medical School (Donald M. Pilisbury, M.D., Director).
This study was supported in part by a grant from the United States
Public Health Service RG 1316(C) and in part by a grant from the
Rockefeller Foundation.
This and preceding papers of this series were
From the also supported in part by U. S. Army Grant #W-49-007-MD-473.
Presented at the Eleventh Annual Meeting of the Society for
Investigative Dermatology,
June 25, 1950 at San Francisco California.
[Note: Plates I-X - description and pictures – that were originally
printed on pages 234-253, are all shown after the text.]
The purpose of the present paper is to draw together and
compare the observations made on the nonpathogenic Nichols strain of
Treponema pallidum both as it occurs in thioglycollate medium (1) and
as it occurs in the embryonated egg (2) under anareobic conditions; the
Kazan (3), Reiter (4), and Noguchi (5) strains of nonpathogenic
Treponema pallidum as they occur in thioglycollate medium; and the
pathogenic Treponema pallidum as it occurs in the experimental
syphiloma in rabbit testes (6, 7). Preliminary observations on Borrelia
anserinum and Borrelia novyi will also be cited (8, 9). The methods of
observation, presented elsewhere (10, 11) have consisted of the use of
phase contrast microscopy and a newly developed stain which has proven
particularly effective in the study of stained impression smears of
infected rabbit testes and in blood films of chickens infected with
Borrelia anserinum, and of rats infected with Borrelia novyi.
The current presentation consists of a synthesis and
correlation of the observations presented in these previous papers. A
brief history of the subject has been presented in conjunction with
paper No. V (1), and will not be recapitulated here, as a general
review of spirochetes and spirochetal diseases is in preparation (12).
OBSERVATIONS
By means of the phase contrast microscope the following
general story of development of spirochetes appears to be consistent in
those organisms studied. The conditions governing the occurrence of the
forms observed and reported are under study. In the current
presentation representative plates from several of these organisms will
be presented in attempting to present the total picture as it has been
observed up to the present time.
Transverse fission. In the organisms so far
studied transverse fission appears to be the most important single
method of vegetative reproduction in spirochetes, especially as they
occur under optimal conditions in their biologic hosts. This process
has been defined by Dobell (13), Zuelzer (14), and others, as the only
mechanism whereby spirochetes reproduce. In Plate I a series of stages
in the transverse division of the Nichols nonpathogenic Treponema
pallidum are presented. Similar observations have been made on the
Kazan, Reiter and Noguchi strains, as well as on the pathogenic
Treponema pallidum, Borrelia novyi, and Borrelia anserinum, as they
occur in the rabbit, rat and chick, respectively. The process of
transverse divisions begins with a rapid bending motion with the bend
always occurring at one point. A break presently appears in the
continuity of the spirochetal body, as shown in Figures 2, 3 and 8,
Plate I. After this break appears, the spirochete undergoes a brief
period of rest and subsequently a lashing motion again ensues. The two
spirochetal fragments, which are still attached by means of the
delicate filament, pull further apart but remain attached to one
another. The delicate membrane holding them together can be observed,
as seen in Figure 4. The organisms then begin a new twisting or spiral
motion in which they literally twist themselves apart and break the
tethering membrane. The long terminal filament so frequently observed
by ourselves (1—5) and many other authors (14), appears to be formed by
the remnant of the membranes which attach the two spirochetes together.
Such terminal filaments are observed in Figures 5 and 7, Plate I.
The point of rupture of the spirochetal body appears to be
associated with the point of attachment or origin of the flagella, as
seen in Figure 8. Recently, by means of improved lighting conditions it
has been possible to follow this process with great clarity, and almost
invariably the point of division is in juxtaposition to the point of
attachment of the flagella.
Transverse division as a means of reproduction is the
single most important method during the early most active phases of
young cultures, and during this period of development, from the first
to about the eighth day in our media, other methods of reproduction are
more difficult to demonstrate. After about the tenth day the
spirochetes begin to elongate and become more sessile, the flagella are
difficult to demonstrate and appear in many instances to be lacking, as
is seen in Figures 1 and 2, Plate IV. At about this same time the
elongating fragments of spirochetes previously produced by transverse
fission begin to form dense granules at any point along their bodies.
Production of gemmae as a means of vegetative reproduction.
The production of gemmae has been observed in all of the organisms
cited above except Borrelia novyi. This organism has not as yet been
adequately studied.
Gemmae, or buds, occur either as dense protuberances with
no inherent structure visible, as demonstrated in Figures 9, 12, 13 and
14, Plate I, or as seen in some of these early bodies, a delicate
limiting membrane can be demonstrated and a dense granule can be
observed, usually attached at the base of these little cysts. Plate II
demonstrates the further production and development of these bodies
while still attached to the parent spirochete, and further demonstrates
that these bodies occur at any point along the spirochetal shaft. The
dense granules which can be visualized within them, as seen in Figure
11, Plate III, tend to elongate into curved rods, as seen in Figures 4,
10, 16 and 14, Plate II. It appears in the present stage of our
observations that the granule that becomes visible within these minute
cysts is the primordium of the daughter spirochete and that the
spirochete is produced by elongation and development of this granule.
Such a process of reproduction is essentially complex, and
it is hoped that future work will make it possible to elaborate on the
details of development.
The gemmae may be dropped off by the parent spirochete at
any stage of their development, as demonstrated by Figures 1, 2 and 3,
Plate III. In Figure i the separation of a short segment of spirochete
with a granule attached is in process. In Figures 2 and 3 such bodies
have separated and in each figure delicate terminal filaments are to be
seen. This is best shown in Figure 3 in which the terminal filament
attached to the granule is observed to be spiral in the same manner as
the parent spirochete. Figures 4—24 show detached spirochetal gemmae in
various stages of development in some of which dense round granules are
visible and in others curved or twisted rods. Figures 26, 27 and 28
demonstrate fairly well differentiated young spirochetes still included
within their cysts, and in Figure 26 especially is the coiled character
of the organism visible. Figures 28, 29 and 30, Plate III, show very
early stages in the emergence of young spirochetes from such
unispirochetal cysts, while Figures 3—7, 9 and 10, Plate IV,
demonstrate later stages in the unipolar emergence of the organisms.
Figures 8 and 11, Plate IV, demonstrate the bipolar emergence of
individual organisms from such unispirochetal cysts and in such
instances the cyst may remain attached to the central portion of the
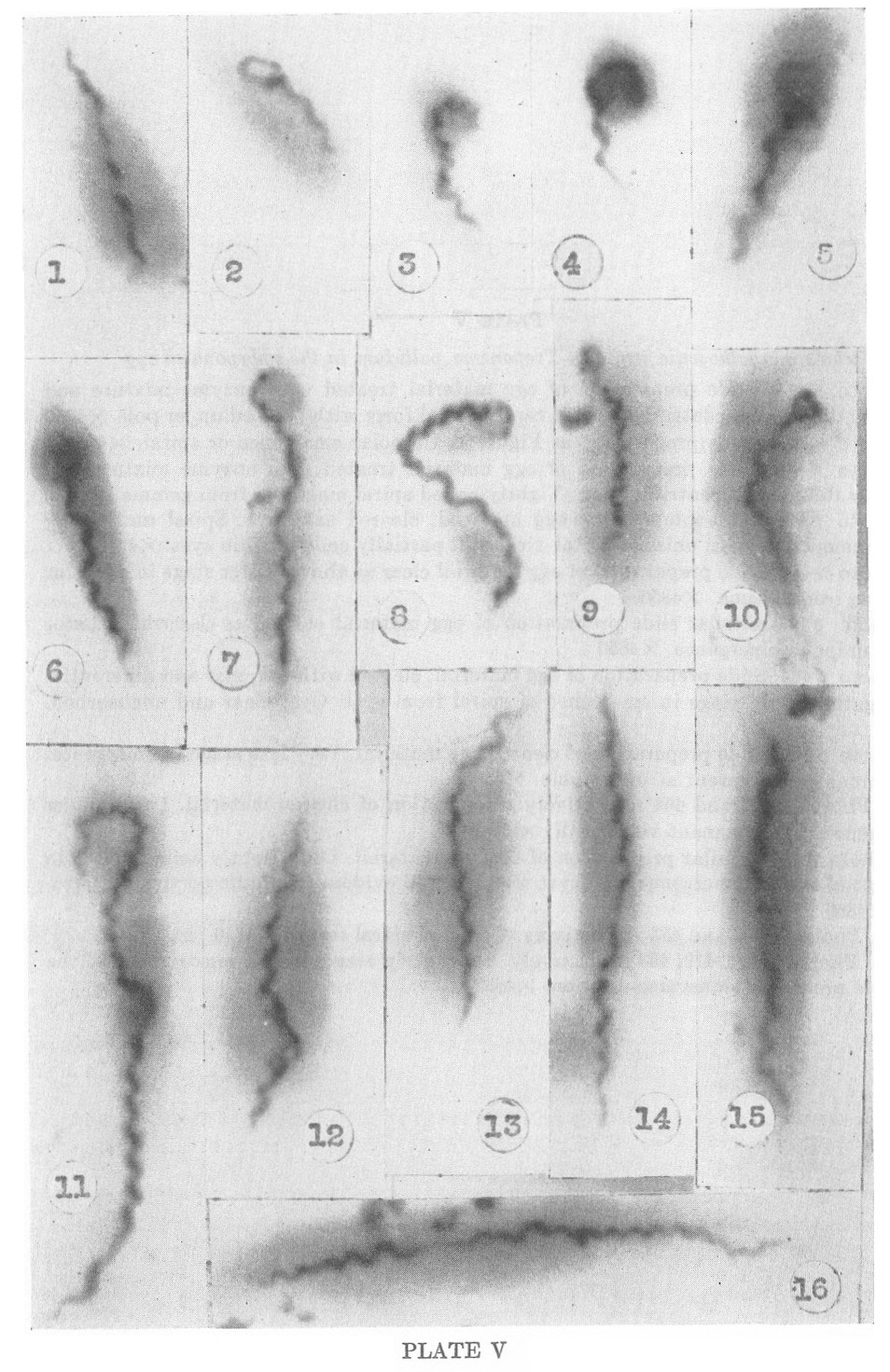
liberated spirochete, as shown in Figure 11, Plate V. Figures 3—10,
Plate V, show the persistence of the originating spirochetal cyst and
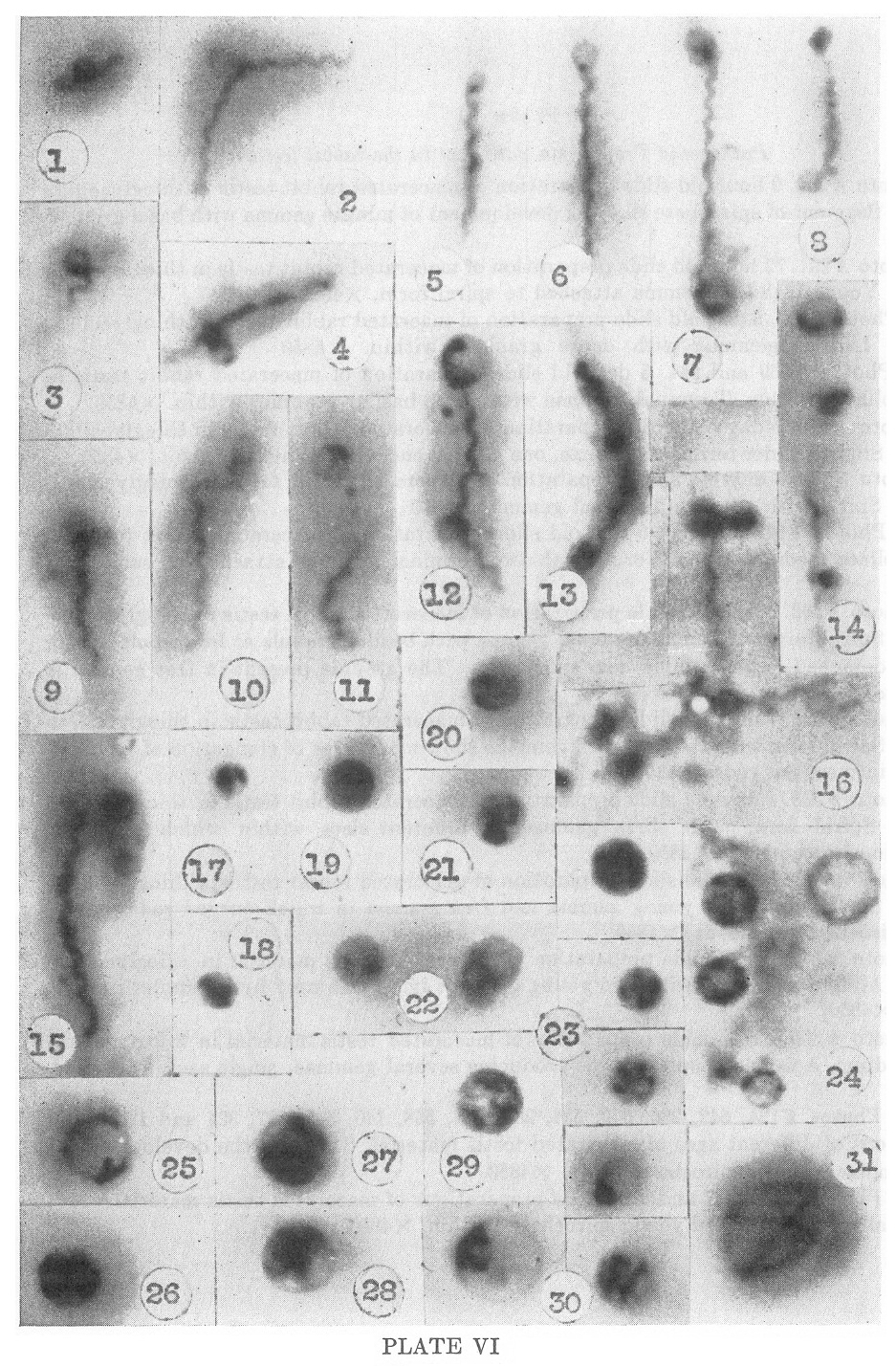
its resorption by the adult liberated organism. Plate VI recapitulates
the process as described for the pathogenic Treponema pallidum as
observed in the rabbit testis.
The production of multispirochetal cysts by the aggregation of organisms.
The following processes have been clearly observed in the Nichols,
Kazan, Reiter and Noguchi strains of nonpathogenic Treponema pallidum,
and in the pathogenic Treponema pallidum. Preliminary observations on
Borrelia novyi and Borrelia anserinum suggest that under certain
conditions similar forms may occur.
An additional method for the reproduction of spirochetes
appears to be by the formation of structures designated here as
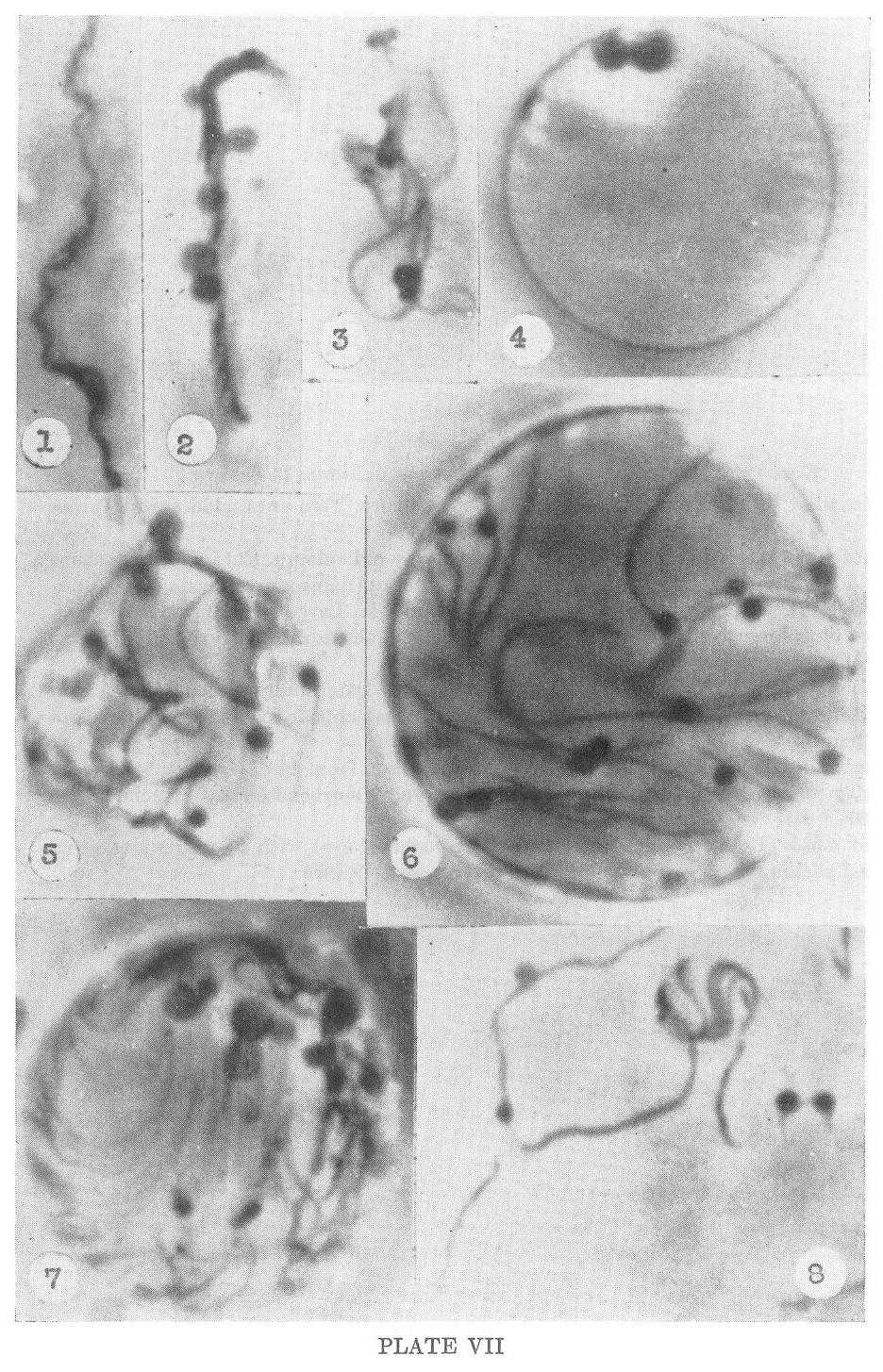
multispirochetal cysts. These appear to be produced in two different
ways. Dense bodies form on clusters of aggregated or paired organisms.
These bodies are denser and larger than those which develop as gemmae
from single spirochetes. They enlarge to considerable size, as
indicated on Plate VII, and a limiting membrane is clearly shown in
Figures 4 and 6. The early stages in the development of these
multispirochetal cysts have yet to be worked out, as under present
conditions of study the internal structures have proven to be too small
for definitive observation. Recent developments in technic, however,
make it appear that it will be possible to study these processes in
more detail. At the present time it can be said that dense granules,
usually lying at one side or at the periphery of the cysts, appear to
reduplicate, forming dense aggregates. From these recognizable
spirochetal filaments develop, and to these granules, which are
currently interpreted as the primordia of individual spirochetes remain
attached, as seen in Figures 5, 6 and 7, Plate VII. In Figure 7 the
central body suggests the possibility that the granules or inclusions,
each of which forms a new spirochete, may reduplicate by a process of
budding. It will be readily seen that these multispirochetal cysts may
obtain tremendous size and may include very large numbers of organisms.
Figure 5, Plate VII, shows such a cyst in which the limiting membrane
has been ruptured. Emergence of adult forms from these large cysts will
be described presently.
The production of multispirochetal cysts by internal reorganization.
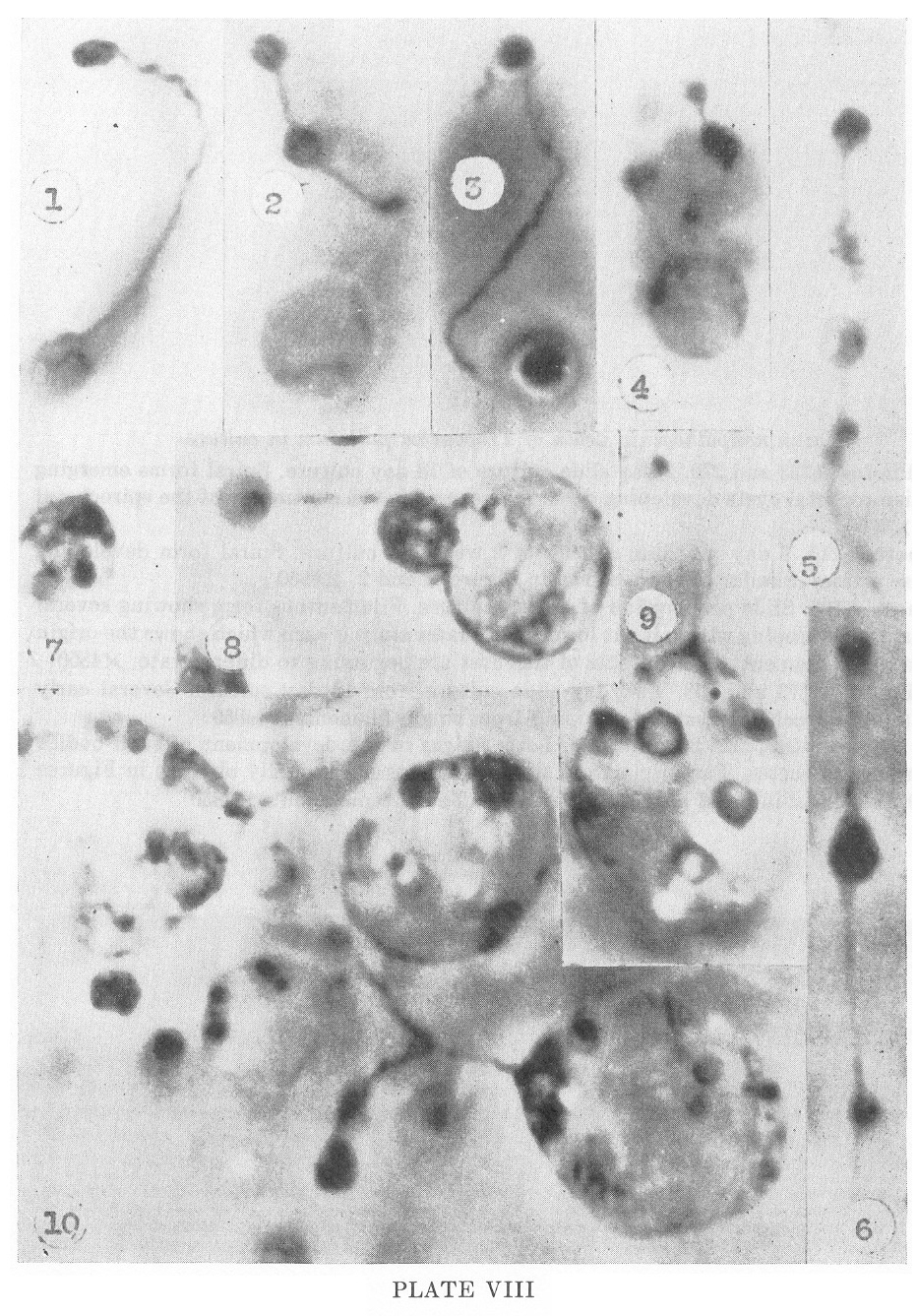
A second means of formation of these large multispirochetal cysts
appears to be by means of reorganization within a single spirochetal
body. Continued observation of these structures suggests that this is
probably the most important method of their formation. Stages in the
development and organization of these structures are seen in Plate
VIII. Figures 2, 5 and 6 show especially clearly the early development
of these bodies from within the continuity of a single spirochete, and
again the early stages of development remain obscure. It is apparent,
however, that the limiting membrane of the spirochete surrounds these
structures and that they are formed from within the continuity of a
single organism. In Figures 9 and 10 particularly, the continuity of
the limiting membrane of the parent spirochete can be observed to form
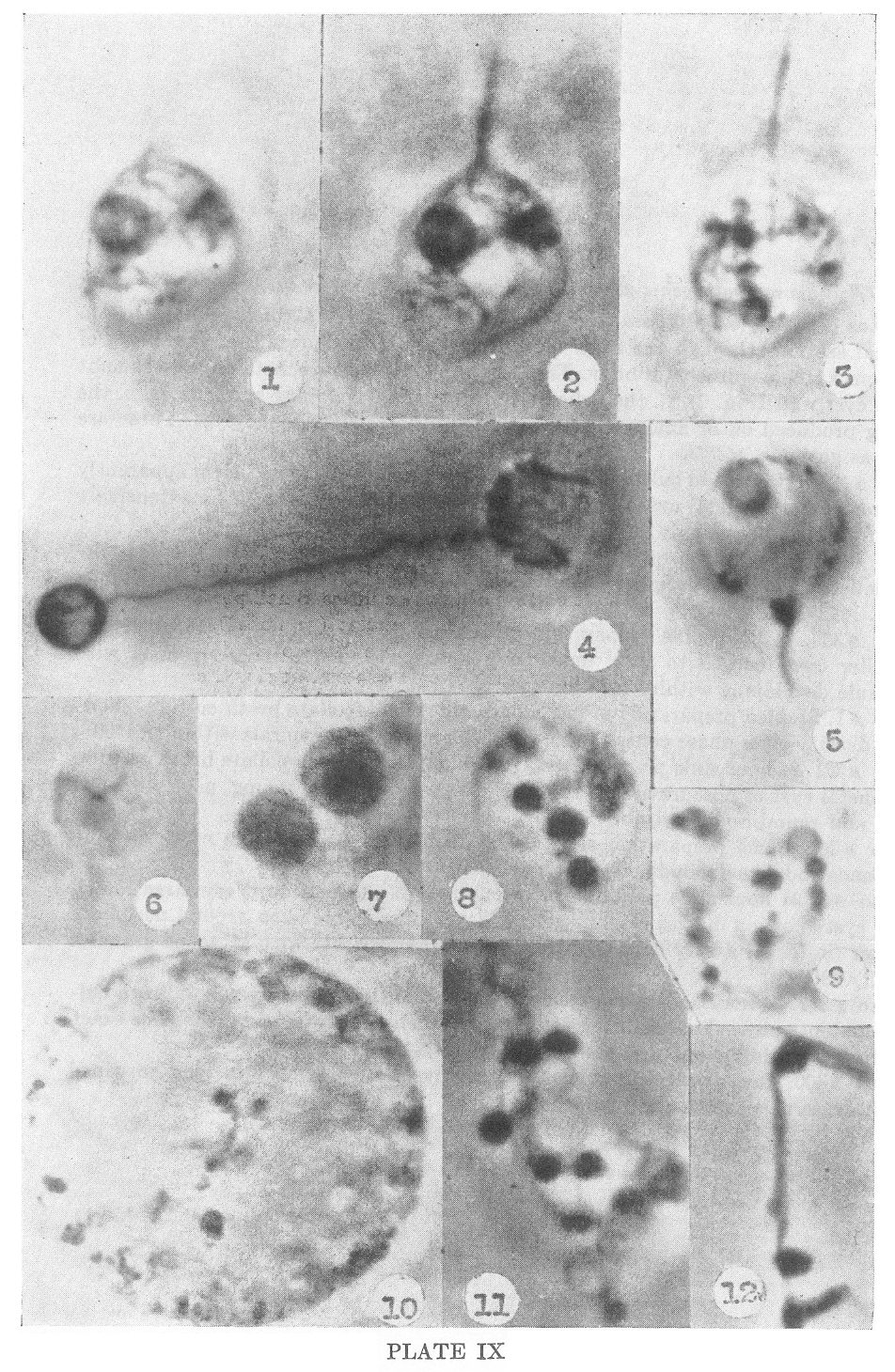
the cyst wall. This is shown in Plate IX, Figures 1, 2 and 3, which are
optical sections through the same multispirochetal cyst. As seen in
Figures 4, 7, 8, 9 and 10 of Plate VIII, these bodies enlarge
tremendously and numerous granules develop within them. Figures 9 and
10 are contents of crushed cysts showing minute granules and cystic
bodies in what appears to be the early developmental stages of the
daughter spirochete. In Figure 10 is shown a large cyst with numerous
inclusions in the center of which, in focus, is a delicate spirochetal
filament attached to a dense granule. Later stages of this
developmental process are again shown clearly in Figures 1, 2 and 3
where the developing spirochetal filaments can be readily observed, and
in Figure 3 the cystic granules are still evident. In Figure 12, Plate
9, is a section of a paired spirochete emerging from a cyst already
forming dense granules which appear to be comparable to the early
stages of development of multispirochetal cysts.
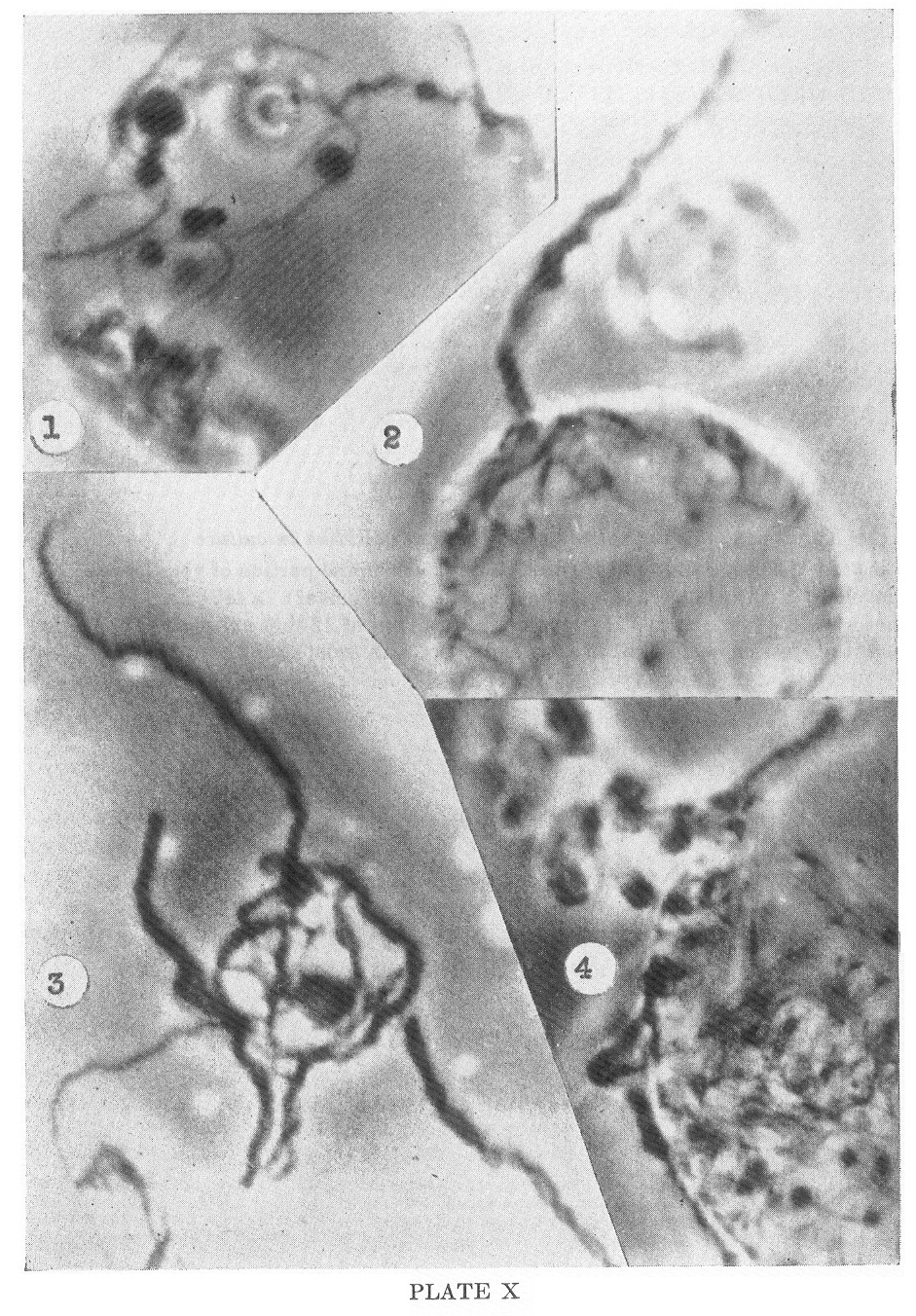
Release of the spirochetes from these multispirochetal
cysts is shown in Plate X, Figures 2, 3 and 4. When mature, the
spirochetes penetrate the cyst wall in massive cords or twisted ropes
and emerge in a manner demonstrated in these figures. Subsequently the
individual spirochetes separate one from another and undergo the
process of segmentation by transverse division and the formation of
unispirochetal gemmae.
The processes which have been described occur in the
cultured forms in old cultures, four to six weeks or more old. In
Treponema pallidum of the pathogenic spirochetes comparable structures
have been observed in material from lesions taken twenty-one or more
days after inoculation.
COMMENT
It is not yet known whether the processes described here are general
for all the spirochetes. Current studies, including observations on
Borrelia novyi and Borrelia anserinum, as well as other saprophytic isolates of Treponema
pallidum suggest that so far as these observations have been taken, we
are dealing with processes of reproduction which apply at least in some
degree in most spirochetes. Further studies will extend these
observations to include many other forms and very possibly modify
current working hypotheses as to the mechanisms of reproduction in
these organisms.
It is hoped that future studies will further elaborate the details of
the internal developmental process of both the unispirochetal and
multispirochetal cysts as well as the details of the association of two
or more organisms in the development of certain of these cysts.
It seems likely that the spirochetes should be considered
as a separate group of microorganisms distinct from the bacteria and
also distinct from the protozoa.
The current problems in the study of these organisms
necessitate the application of all available methods, including the
production of special staining methods, the further application of
electron microscopy, and the continued application, with modification,
of phase contrast microscopy. The most important single feature of this
latter technic appears to be the use of adequate illumination,
preferably of a monochromatic character.
Current studies must be considered at the present time to
be preliminary. Further development of the subject must depend upon a
continued accumulation of data and observations, and particularly upon
the development of pure cultural technics for the pathogenic forms so
that these may be studied under more highly controlled conditions.
REFERENCES
(1) DELAMATER, E. D., HAANES, M., AND WIGGALL, R., 1951: Studies on the
Life Cycle of Spirochetes. V. The Life Cycle of the Nichols
Nonpathogenic Treponema pallidum in Culture. Am. Jour. Syph., Gonorh.
& Ven. Dis. (in press)
(2) DELAMATER, E. D., HAANES, M., AND WIGGALL, R., 1951: Studies on the
Life Cycle of Spirochetes. VI. The Life Cycle of the Nichols
Nonpathogenic Treponema in duallsth pe Embryonated Hen’s Egg. Am. Jour.
Syphilis, Gonorh. & V. D. (in press)
(3) DELAMATER, E. D., HAANES, M., AND WIGGALL, R., 1951: Studies on the
Life Cycle of Spirochetes. VII. The Life Cycle of the Kazan
Nonpathogenic Treponema pallidum in Culture. Am. Jour. Syph., Gonorh.
& Ven. Dis. (in press)
(4) DELAMATER, E. D., HAANES, 1sf., AND WIGALL, R., Studies on the Life
Cycle of Spirochetes. Evidence for a Similar Life Cycle in the Reiter
Nonpathogenie Treponema pallidum in Culture.
(5) DELAMATER, E. D., HAANE5, M., AND WIGGALL, R., Studies on the Life
Cycle of Spirochetes. The Occurrence of Complex Growth Processes in the
Noguchi Nonpathogenic Treponema pallidum in Culture. (In preparation.)
(6) DELAMATER, E. D., WIGGALL, R., AND HAANES, M., 1950: Studies on the
Life Cycle of Spirochetes. III. The Life Cycle of the Nichols
Pathogenic Treponema pallidum in the Rabbit Testis as Seen by Phase
Contrast Microscopy. J. Exper. Med. 92: 239—246.
(7) DELAMATER, E. D., WIGGALL, R., AND HAANES, M., 1950: Studies on the
Life Cycle of Spirochetes. IV. The Life Cycle of the Nichols Pathogenic
Treponema pallidum in the Rabbit Testis as Visualized by means of
Stained Smears. J. Exper. Med. 92: 247—252.
(8) DELAMATER, E. D., AND HAANE5, M.: Studies on the Life Cycle of
Spirochetes. Observations on the Life Cycle of Borrelia anserinum. (In
preparation.)
(9) DELAMATER, E. D.. AND HAANES, M.: Studies on the Life Cycle of
Spirochetes. Observations on the Occurrence of a Life Cycle in Borrelia
novyi. (In preparation.)
(10) DELAMATER, E. D., HAANES, M., AND WIGGALL, R., 1950: Studies on
the Life Cycle of Spirochetes. I. The Use of Phase Contrast Microscopy.
Am. J. Syph., Gonor. & Ven. Dis. 34: 122—125.
(11) DELAMATER, E. D., HAANES, M., AND WIGGALL, R., 1950: Studies on
the Life Cycle of Spirochetes. II. The Development of a New Strain. Am.
J. Syph., Gonor. & Ven. Dis. 84: 515—518.
(12) DELAMATER, E. D., URBACH, F., AND HAANES, M. : Studies on the Life
Cycle of Spirochetes. Historical Review and Correlation with Current
Studies. (In preparation.)
(13) DOBELL, C. C., 1912: Researches on the Spirochetes and Related Organisms, Arch. f. Protistenk 26: 117—240.
(14) ZUELZER, M., 1925: Die Spirocheten. Handbuch der Pathogenen Protozoen 11: 1627— 1797.
DISCUSSION
DR. ARTHUR C. CURTIS: I think the work of Dr. DeLamater
and his coworkers is a major scientific contribution. It is interesting
to look at the cyst-like bodies of the spirochete he has shown and
wonder whether some might not be resting forms of the organism. Dr.
Wile has for many years believed a “spore-like” or resting form of
syphilis occurs. We have done experimental studies in mouse syphilis
and have been unable to find spirochetes after exhaustive darkfield
search throughout their brain tissue, yet after inoculation of this
material into rabbit testicles syphilis occurs. It may be possible that
these cyst-like bodies instead of spirochetes were present and when
they are put into a more favorable medium, they again develop into
spirochetes and produce the disease.
DR. STEPHEN ROTHMAN: I would like to ask the authors
whether the formation of cysts, development of spirochetes in the cysts
and their breaking through the wall has been directly observed, or
whether the cycle is reconstructed from single still pictures as
observed in the phase microscope.
DR. S. WILLIAM BECKER: I am sure that those who saw Doctor
DeLamater’s exhibit at the Academy in December were very much impressed
by it. I think it should be explained that under the phase microscope
you see first highly refractive bodies followed by red ones which can
be very well identified. Certainly these pictures are the best method
of visualizing the spirochete.
DR. DONALD M. PILLSBURY: We have had the same feeling as
Doctor Curtis; certainly in organisms fresh out of tissue they tend to
divide by transverse fission and form small gemmae and large cysts. It
is hard to say that it represents one spirochete. In spite of the hot
summer, there is a project on to take moving pictures and we may get a
little better idea of what actually occurs.
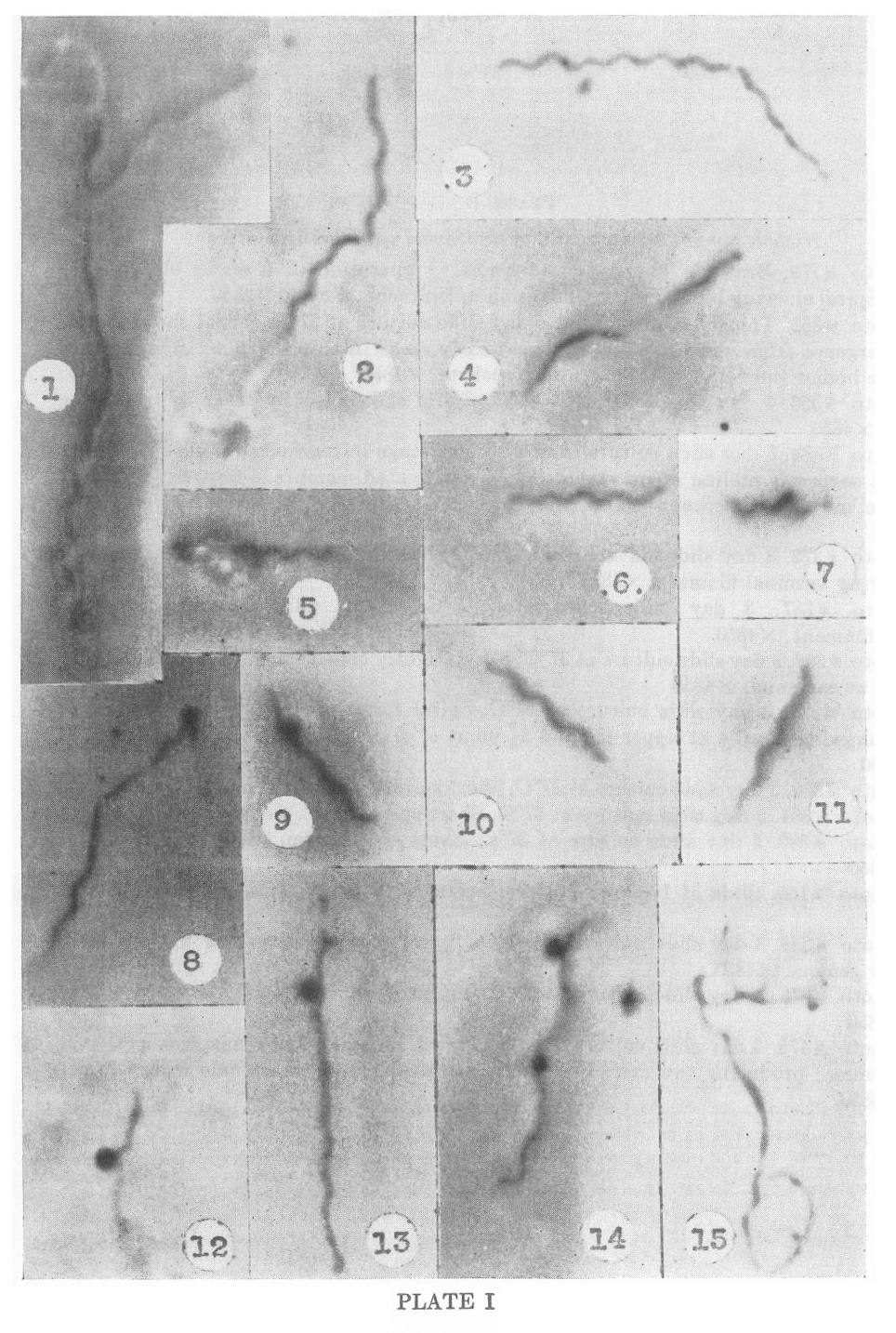
PLATE I
Nichols nonpathogenic strain of Treponema pallidum in culture
1. Photo #179. Brewer’s Thioglycollate media. Preparation of 6 weeks
old cultures at 37°C. Spiral showing bipolar emergence from
unispirochetal cyst. X4850
2. Photo #352. Thioglycollate media. 1 day slide culture at 37°C.
Spiral showing unipolar emergence from unispirochetal cyst and early
stage in transverse division. Several chromatic bodies (nuclei?)
visible within spirochete. X4850
3. Photo #356. 1 day slide culture at 37°C. Spiral showing early stage in transverse division. X4850
4. Photo #388. 3 day slide culture at 37°C. Later stage in transverse
division showing two short segments pulling apart with delicate,
attenuated membrane between them. At this stage movement becomes spiral
and spirochetes literally twist themselves apart. X4850
5. Photo #372. 3 day slide culture at 37°C. Spiral partly emerged from unispirochetal cyst showing terminal filament. X4850
6. Photo #387. 3 day slide culture at 37°C. Short spiral segment showing terminal filament. X4850
7. Photo #398. 3 day slide culture at 37°C. Short, tightly coiled spiral form with delicate filaments at each end. X4850
8. Photo #374. 3 day slide culture at 37°C. Spiral form in early stage
of transverse division showing flagella at upper tip and at point of
division. Gemma forming at upper tip. X4850
9. Photo #390. 3 day slide culture at 37°C. Short spiral forming a gemma or bud. X4850
10. Photo #384. 3 day slide culture at 37°C. Short spiral form. X4850.
11. Photo #390. 3 day slide culture at 37°C. Short spiral form. (Same photoplate as 49.) X4850
12. Photo #165. Slide of 1 month culture. Spiral with small dense gemma attached. X4850
13. Photo #383. 3 day slide culture at 37°C. Spiral showing formation of very early and older gemma. X4850
14. Photo #378. 3 day slide culture at 37°C. Spiral form developing two dense gemmae. X4850
15. Photo #475. 5 day slide culture at 37°C. Spiral emerging from
unispirochetal cyst (adult gemma) producing two early daughter gemmae.
Note dense granule within larger form. X4850
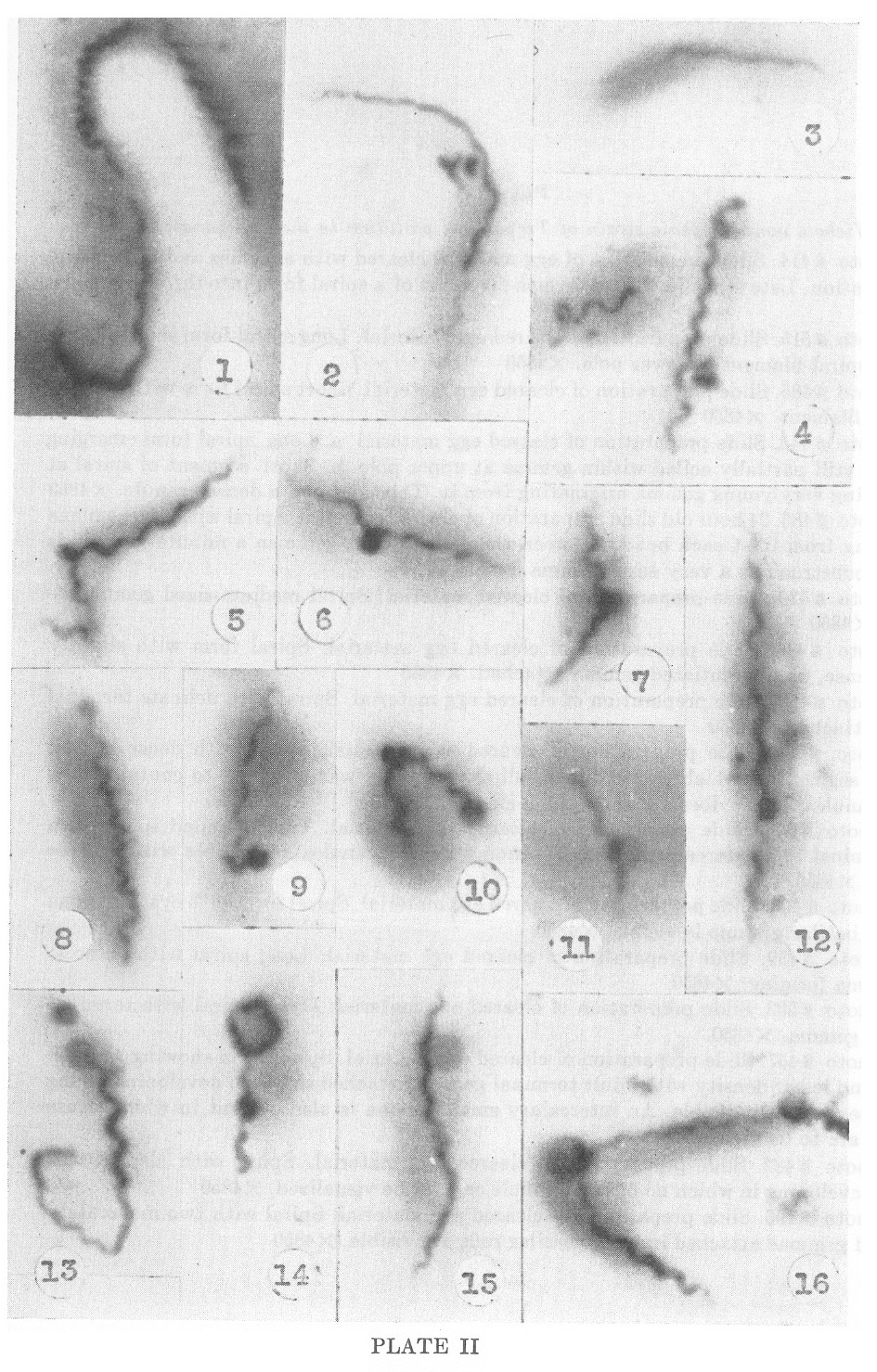
PLATE II
Nichols nonpathogenic strain of Treponema pallidum in the embryonated egg
1. Photo #414. Slide preparation of egg material cleared with enzymes
and differential centrifugation. Late stage in the transverse diversion
of a spiral form into three segments. X4850
2. Photo #515. Slide preparation of cleared egg material. Long spiral
form showing long delicate spiral filament at lower pole. X4850
3. Photo #435. Slide preparation of cleared egg material. Short spiral form with delicate terminal filament. X4850
4. Photo #445. Slide preparation of cleared egg material. a. Long
spiral form emerging from and still partially coiled within gemma at
upper pole. b. Short segment of spiral at left showing very young gemma
originating from it. This contains a dense granule. X4850
5. Photo #486. 24 hour old slide preparation of cleared material.
Spiral with two gemmae originating from it at each bend. Between these
two larger gemmae a minute granule is present, construed as a very
early gemma. X4850
6. Photo #473. Slide preparation of cleared material. Spiral medium-sized gemma attached. X4850.
7. Photo #446. Slide preparation of cleared egg material. Spiral form
with slightly larger, dense, undifferentiated gemma attached. X4850
8. Photo #450. Slide preparation of cleared egg material. Spiral with delicate terminal gemma attached. X4850
9. Photo #454. Slide preparation of cleared egg material. Spiral with
dense curved terminal segment of which the terminal ball-shaped body
was observed to contain three dense granules. Photo does not show these
clearly. X4850.
10. Photo #438. Slide preparation of cleared egg material. Tightly
coiled spiral form with terminal and intercalary gemmae forming dense
granules are visible within these gemmae. X4850
11. Photo #404. Slide preparation of cleared egg material. Spiral form
with cystic gemma in which basilar granule is visible. X4850
12. Photo #459. Slide preparation of cleared egg material. Long spiral with intercalary gemma forming. X4850
13. Photo #502. Slide preparation of cleared egg material. Dense spiral with terminal stipitate gemma. X4850.
14. Photo #457. Slide preparation of cleared egg material. Spiral form
showing areas of greater and Jesser density with adult terminal gemma
attached in which developing young spirochete is clearly visible. An
intercalary small gemma is also present iii which dense granules are to
be seen.
15. Photo #482. Slide preparation of cleared egg material. Spiral with
clear cystic gemma developing in which no basilar granule can yet be
visualized. X4850
16. Photo #496. Slide preparation of cleared egg material. Spiral with
two moderately advanced gemmae attached iii which basilar rods are
visible. X4850
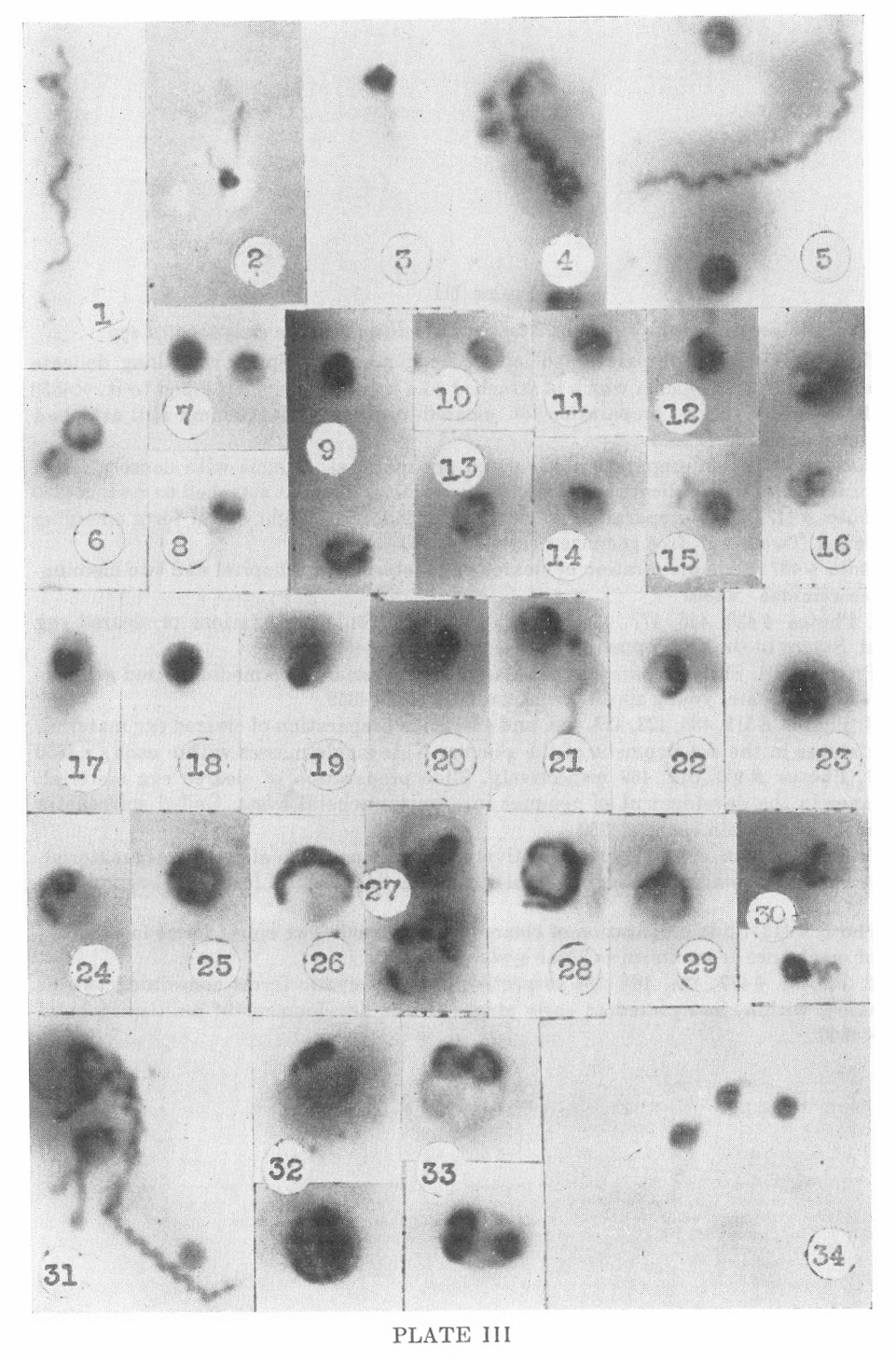
PLATE III
Nichols nonpathogenic strain of Treponema pallidum in the embryonated egg
1. Photo #478. Slide preparation of cleared egg material. Spiral with
long delicate terminal filament with gemma which is attached to a
spiral segment attached to it. X4850
2. Photo #405. Slide preparation of cleared egg material. Gemma still attached to fragment of spiral. X2425
3. Photo #509. Slide preparation of cleared egg material. Gemma with
densely coiled spiral form within, with delicate spiral similar to
terminal filament attached to cyst. X4850
4. Photo #417. Slide preparation of cleared egg material. Tight spiral
form emerging from gemma. Two small, free gemmae near spiral. X4850
5. Photo #437. Slide preparation of cleared egg material. Adult spiral and two mediumsized free gemmac. X4850
6-15. Photos #430, 426, 477, 431, 426, 430, and 422. Slide preparations
of cleared egg material. Stages in the development of freed gemmae.
X4850
16. Photo #404. Slide preparation of cleared egg material. Two
medium-sized gemmae within which delicate, young spiral forms can be
seen. X4850
17-24. Photos #511, 488, 423, 426, 488, and 484. Slide preparation of
cleared egg material. Further stages in the development of the gemma.
Note single masses within each. X4850
25-27. Photos #406, 512, 469 respectively. Slide preparation of cleared
egg material. Late stages in the development of gemmae into
unispirochetal cyste. Coiled spirochetes can be made out within each.
X4850
28-30. Photos #516, 426, 511, 409 respectively. Slide preparation of
cleared egg material. Late developmental stages of unispirochetal cyste
from which spiral forms are beginning to emerge. X4850
31. Photo #413. Slide preparation of cleared egg material. Two spiral
forms in different stages of emergence from unispirochetal cysts. X4850
32-34. Photos #477, 424, 464, 354 respectively. Small cystic forms
containing two or more masses within, interpreted as early stages in
the development of multispirochetal cyste. X4850
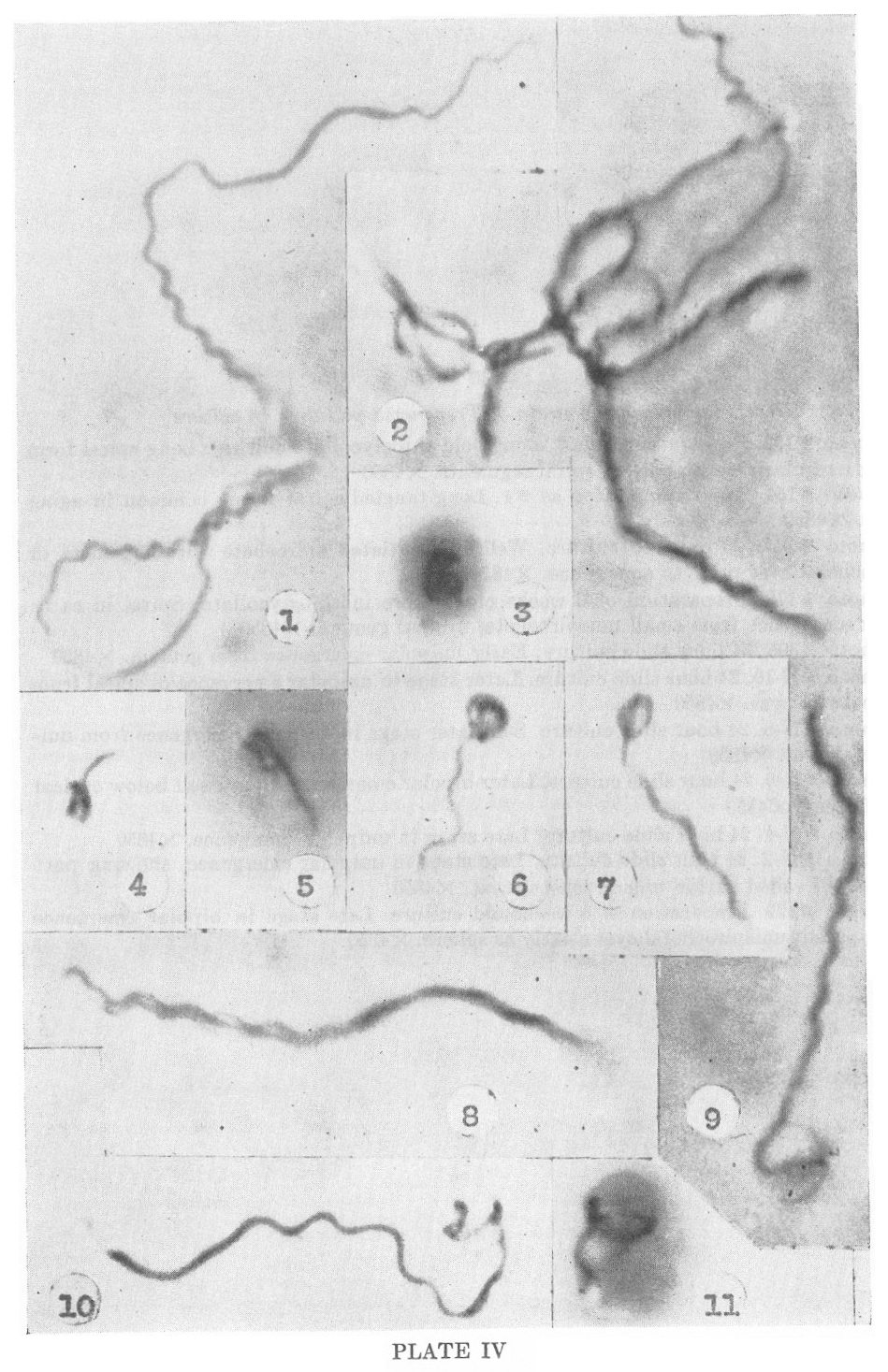
PLATE IV
Nichols nonpathogenic strain of Treponema pallidum in culture
1. Photo #152. Preparation from 1 month old thioglycollate culture.
Long spiral form showing irregularly and regularly spiral segments.
X4850
2. Photo #154. Same preparation as #1. Long tangled spiral forms common in aging cultures. X4850
3. Photo #357. 23 day old culture. Well-differentiated spirochete within gemma or unispirochetal cyst prior to emergence. X4850
4. Photo #179. Preparation of 6 weeks old culture in thioglycollate.
Spiral in early unipolar emergence from small unispirochetal cyst or
gemma. X4850
5. Photo #395. 24 hour slide culture. Early unipolar emergence from gemma. X4850
6. Photo #N-10. 24 hour slide culture. Later stage in unipolar emergence of spiral from unispirochetal cyst. X4850
7. Photo #N-8. 24 hour slide culture. Still later stage in unipolar emergence from unispirochetal cyst. X4850
8. Photo #N-6. 24 hour slide culture. Later bipolar emergence. Cyst itself below optical plane of focus. X4850
9. Photo #N-4. 24 hour slide culture. Late stage in unpolar emergence. X4850
10. Photo #N-2. 24 hour slide culture. Late stage in unipolar
emergence, showing part of spiral still coiled within unispirochetal
cyst. X4850
11. Photo #179. Preparation of 6 weeks old culture. Late stage in
bipolar emergence showing parent unispirochetal cyst clearly as sphere.
X4850
PLATE V
Nichols nonpathogenic strain of Treponema pallidum in the embryonated egg
1. Photo #474. Slide preparation of egg material treated with enzyme
mixture and cleared by differential centrifugation. Average spiral form
with pointed upper pole. X4850
2. Photo #478. Same preparation as Figure 1. Unipolar emergence or spiral. X4850’
3. Photo #448. Slide preparation of egg material treated with enzyme
mixture and cleared by differential centrifugation. Tightly coiled
spiral emerging from gemma. X4850
4. Photo #500. Slide preparation egg material, cleared as above. Spiral
undergoing unipolar emergence from unispirochetal cyst. Still partially
coiled within cyst. X4850
5. Photo #441. Slide preparation of egg material clear as above. Later stage in unipolar emergence from gemma. X4850
6. Photo #443. Similar slide preparation of egg material cleared as described. Later stage in unipolar emergence. X4850
7. Photo #463. Slide preparation of egg material, cleared with enzymes
and differential centrifugation. Late stage in emergence of spiral from
cyst. Cyst clear and unabsorbed. X4850
8. Photo #460. Slide preparation of cleared egg material. Very late
stage in emergence. Cyst remnant sill present at upper pole. X4850
9-10. Photos #457 and 464 respectively. Preparation of cleared
material. Later stages in emergence. Cyst remnant very small. X4850
11. Photo #498. Similar preparation of cleared material. Long tightly
coiled spiral in final stage of bipolar emergence with cyst remnant
still evident in middle portion of spirochete. X4850
12-13. Photos #473 and 483 respectively. Common spiral forms. X4850
14-16. Photos #454, 419, 453 respectively. Show early stages in the
construction of the spirochete preceding transverse division. X4850
PLATE VI
Pathogenic Treponema pallidum in the rabbit testis
1. Photo #296. 9 hour old slide preparation of macerated rabbit testis
in thioglycollate medium. Segment of spirochete showing development of
minute gemma with basal granule. X4850
2. Photo #291. 72 hour old slide preparation of macerated rabbit testis
in thioglycollate medium. Young bleb-like gemma attached to spiral
form. X4850
3, 4. Photo #522. 5 day old slide preparation of macrated rabbit testis
in thioglycollate medium. Lateral gemmae with dense granules within.
X4850
5, 6. Photos #539 and 544. 5 day old slide preparation of macerated
rabbit testis in thioglycollate medium. Terminal gemmae with dense
basilar granules within. X4850
7. Photo #572. 7-day old slide preparation of macerated rabbit testis
in thioglycollate medium. Slightly older terminal gemmae, one at each
end with granules present. X4850
8. Photo # 339. 3 day old slide preparation of macerated rabbit testis
in thioglycollate medium. Spiral with stipitate terminal gemma. X4850
9, 10. Photos # 348 and 330. 5 day old slide preparation of macerated
rabbit testis in thioglycollate medium. Spiral forms with two terminal
gemmae attached at same end. X4850
11. Photo #563. 5 day old slide preparation of macerated rabbit testis
in thioglycollate medium. Spiral form with small terminal gemma with
basilar granule at lower pole, and a recently detached larger gemma
near upper pole. The granule present in free gemma is seen to be
elongating. X4850
12. Photo #574. 7 day old slide preparation of macerated rabbit testis
in thioglycollate medium. Spiral form with two attached gemmae showing
degrees of elongation of included granules into curved rods. X4850
13. Photo #533. 4 day old slide preparation of macerated rabbit testis
in thioglycollate medium. Spiral form with three gemmae of different
sizes within which early differentiation is occurring. X4850.
14. Photo #581. 5 day old slide preparation of macerated rabbit testis
in thioglycollate medium. Spiral form with young gemma and free gemma
mi which curved rod forming young spirochete is evident. X4850
15. Photo #547. 5 day slide preparation of macerated testis material in
thioglycollate medium. Adult spiral form with two young gemmae lying
free near by. Granules present in each. X4850
16. Photo #305. Fresh slide preparation of macerated testis material in
thioglycollate broth medium. A tangle of spiral forms producing several
gemmae, single and in clusters. X4850
17-29. Photos #124, 542, 286, 549, 534, 288, 541, 558, 14), 565, 537,
324 and 123. Slide preparations of different ages of macerated testis
material. Stages in the development of freed gemmae into unispirochetal
cysts. X4850
30-31. Photos #569, 537 and 529. Slide preparations of macerated testis
material. Unispirochetal cysts with coiled young spirochetes within.
X4850
PLATE VII
Kazan nonpathogenc strain of Treponema pallidum in culture
1. Photo #201. Slide preparation of 11 day old culture. Two entangled spiral forms. X4850
2-3. Photos #200 and 191. Slide preparation of 11 day old culture.
Entangled organisms producing dense masses which appear to eventuate
into multispirochetal cysts. X4850
4. Photo #194. Slide preparation of 11 day old culture. Large
multispirochetal cyst. Early stage of differentiation. Double mass at
top continues multiplication and differentiation. Wall of cyst clearly
shown. X4850
5. Photo #204. Slide preparation of 11 day old culture. Later
multispirochetal cyst that has been ruptured showing developing
spirochetes and what appear to be gemmae developing from them. X4850
6-7. Photos #212 and 220. 3 day old slide culture made from 14 day old
culture. Very large multispirochetal cysts showing entangled developing
spirochetes and attached gemmae. X4850
8. Photo #526. (Reiter Strain) Smaller multispirochetal cyst with
filaments emerging. Dense spiral within cyst. No gemmae present within
cyst. X4850
PLATE VIII
Kazan nonpathogenic strain of Treponema pallidum in culture
1-2. Photos #233 and 270. 2 day slide culture of 18 day culture. Spiral
forms emerging from unispirochetal cysts developing dense masses within
the continuity of the spirochetal bodies. X4850
3. Photo #215. 3 day old slide culture of 2 week old culture. Spiral
form developing two dense masses. Similar to those shown in Figures i
and 2. X4850
4. Photo #196. Slide preparation of 12 day culture. Filamentous form
showing several dense masses developing within it. At lower pole a
later stage is seen which shows the origin from the single filament.
The contents of this cyst are beginning to differentiate. X4850
5-6. Photos #273 and 268. Two day slide culture from 18 day culture.
Several early masses (multispirochetal cysts) developing from single
filaments. X4850
7-10. Photos #247, 222, 206, and 266. Later stages in the development
of such bodies from single spirochetes. The origin from single
filaments is especially obvious in Figures 9 and 10. Details of
internal differentiation are difficult to make out. X4850
PLATE IX
Nichols nonpathogenic strain of Treponema pallidum in culture
1-3. Photos #56, 58, 59. Preparation of 6 weeks old culture in
thioglycollate broth. Three optical sections through the same
multispirochetal cyst showing: a. the Origin of the cysts from a single
spirochetal body. The membrane of the spirochete appears to split to
form the cyst wall (Fig. 2). b. the delicate fibrils of the developing
spirochetes. c. the bodies being produced on or among these young
spirochetes within the cyst. These are interpreted as gemmae. X4850
4. Photo #187. 6 weeks old culture in thioglycollate broth. Long spiral
form apparently emerging from unispirochetal cyst at right, within
which it can be seen to be extensively coiled, and forming a dense body
at opposite pole. X4850
5. Photo #160. Preparation of one month old culture in thioglycollate
broth. Large cyst wall; delicate spirals at periphery of cyst cut in
cross section, and mass developing within cyst. X4850
6. Photo #369. 24 hour slide preparation of one month old culture in
thioglycollate broth. Smaller cyst focused to show developing
spirochete and attached gemma with basilar granule developing within.
X4850
7. Photo #1. Stained preparation of one month old thioglycollate broth
culture, showing greater density than phase contrast preparations and
delicate spirals within. X4500
8. Photo #361. 24 hour slide preparation of one month old
thioglycollate broth culture. Multispirochetal cyst of obscure origin,
showing cyst wall and very young, poorly-defined spirochetes and round
bodies within. X4850
9. Photo #363. Same preparation as 8. Cyst contents pressed from cyst, showing delicate strands and dense round bodies. X4850
10. Photo #3. 24 hour slide preparation of one month old culture. Very
large multispirochetal cyst showing masses of very young, developing
spirochetes around periphery and in center (in focus) a very delicate
irregular young spirochete with attached granules (gemmae?). X4850
11. Photo #371. 24 hour slide preparation of a one month old
thioglycollate broth culture. Cyst contents pressed out showing dense
masses, delicate fibrils and blebs. The exact nature and organization
is obscure. X4850
12. Photo #400. One month old culture in thioglycollate broth. Two long entwined spirals with dense masses attached. X4850
PLATE X
Kazan nonpathogenic strain of Treponema pallidum in culture
1. Photo #236. 3 day old slide culture of 12 day culture. Small portion
of very large cyst showing developing spirochetes and attached granules
(gemmae?). X4850
2-4. Photos #217, 209, and 225. 3 day old slide culture of 12 day
culture. Very large multispirochetal cysts showing emergence of
spirochetes in twisted cords. X4850